Designing hydrogels for controlled drug delivery
- PMID: 29657852
- PMCID: PMC5898614
- DOI: 10.1038/natrevmats.2016.71
Designing hydrogels for controlled drug delivery
Abstract
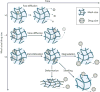
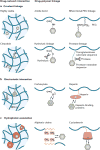
Hydrogel delivery systems can leverage therapeutically beneficial outcomes of drug delivery and have found clinical use. Hydrogels can provide spatial and temporal control over the release of various therapeutic agents, including small-molecule drugs, macromolecular drugs and cells. Owing to their tunable physical properties, controllable degradability and capability to protect labile drugs from degradation, hydrogels serve as a platform in which various physiochemical interactions with the encapsulated drugs control their release. In this Review, we cover multiscale mechanisms underlying the design of hydrogel drug delivery systems, focusing on physical and chemical properties of the hydrogel network and the hydrogel-drug interactions across the network, mesh, and molecular (or atomistic) scales. We discuss how different mechanisms interact and can be integrated to exert fine control in time and space over the drug presentation. We also collect experimental release data from the literature, review clinical translation to date of these systems, and present quantitative comparisons between different systems to provide guidelines for the rational design of hydrogel delivery systems.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures



References
-
- Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. - PubMed
-
- Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49:1993–2007.
-
- Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908–908. - PubMed
-
- Florence AT, Jani PU. Novel oral drug formulations. Drug Safety. 1994;10:233–266. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
