Method for the Culture of Mouse Pulmonary Microvascular Endothelial Cells
- PMID: 29658013
- PMCID: PMC5898805
Method for the Culture of Mouse Pulmonary Microvascular Endothelial Cells
Abstract
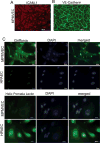
Pulmonary microvascular endothelial cells (ECs) are integral to the alveoli-capillary barrier of the lung. The EC barrier integrity is known to be disrupted in severe lung diseases such as acute respiratory distress syndrome (ARDS), pneumonia and pulmonary edema. Mice are commonly used to model these diseases, dictating an increasingly high demand for murine ECs isolation and culture. Despite the significant number of protocols for the culture of various types of murine cells, the isolation of microvascular endothelial cells remains a challenging procedure. In our manuscript we developed adetailed step-by-step refined method for isolation murine pulmonary microvascular ECs for in vitro studies. We separated cells using platelet endothelial cell adhesion molecule antibody and characterized ECs with antibodies against intercellular adhesion molecule-1, acetylated-low density lipoprotein, and vascular endothelial (VE)-cadherin. Further, we confirmed microvascular origin of these cells using Griffonia simplicifolia and Helix pomatia (negative control) staining. Barrier properties of EC monolayer were characterized by conducting electric cell-substrate impedance sensing experiments with the edemagenic agents, lipopolysaccharide and nocodazole, and known barrier-protective agents, adenosine and sphingosine-1-phosphate. The described complete protocol provided consistent and reproducible results.
Keywords: Cell culture; Lung; Method; Microvascular endothelial cells; Murine pulmonary endothelial cells.
Conflict of interest statement
Disclosure/Duality of Interest No author has any disclosure or duality of interest for any product or result in this manuscript.
Figures






References
-
- Akis N, Madaio MP. Isolation, culture, and characterization of endothelial cells from mouse glomeruli. Kidney Int. 2004;65:2223–2227. - PubMed
-
- Wu Z, Hofman FM, Zlokovic BV. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J Neurosci Methods. 2003;130:53–63. - PubMed
-
- Marelli-Berg FM, Peek E, Lidington EA, et al. Isolation of endothelial cells from murine tissue. J Immunol Methods. 2000;244:205–215. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
