Genioglossus reflex responses to negative upper airway pressure are altered in people with tetraplegia and obstructive sleep apnoea
- PMID: 29658103
- PMCID: PMC6046082
- DOI: 10.1113/JP275222
Genioglossus reflex responses to negative upper airway pressure are altered in people with tetraplegia and obstructive sleep apnoea
Abstract
Key points: Protective reflexes in the throat area (upper airway) are crucial for breathing. Impairment of these reflexes can cause breathing problems during sleep such as obstructive sleep apnoea (OSA). OSA is very common in people with spinal cord injury for unknown reasons. This study shows major changes in protective reflexes that serve to keep the upper airway open in response to suction pressures in people with tetraplegia and OSA. These results help us understand why OSA is so common in people with tetraplegia and provide new insight into how protective upper airway reflexes work more broadly.
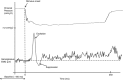
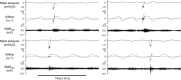
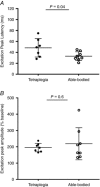
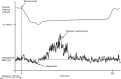
Abstract: More than 60% of people with tetraplegia have obstructive sleep apnoea (OSA). However, the specific causes are unknown. Genioglossus, the largest upper-airway dilator muscle, is important in maintaining upper-airway patency. Impaired genioglossus muscle function following spinal cord injury may contribute to OSA. This study aimed to determine if genioglossus reflex responses to negative upper-airway pressure are altered in people with OSA and tetraplegia compared to non-neurologically impaired able-bodied individuals with OSA. Genioglossus reflex responses measured via intramuscular electrodes to ∼60 brief (250 ms) pulses of negative upper-airway pressure (∼-15 cmH2 O at the mask) were compared between 13 participants (2 females) with tetraplegia plus OSA and 9 able-bodied controls (2 females) matched for age and OSA severity. The initial short-latency excitatory reflex response was absent in 6/13 people with tetraplegia and 1/9 controls. Genioglossus reflex inhibition in the absence of excitation was observed in three people with tetraplegia and none of the controls. When the excitatory response was present, it was significantly delayed in the tetraplegia group compared to able-bodied controls: excitation onset latency (mean ± SD) was 32 ± 16 vs. 18 ± 9 ms, P = 0.045; peak excitation latency was 48 ± 17 vs. 33 ± 8 ms, P = 0.038. However, when present, amplitude of the excitation response was not different between groups, 195 ± 26 vs. 219 ± 98% at baseline, P = 0.55. There are major differences in genioglossus reflex morphology and timing in response to rapid changes in airway pressure in people with tetraplegia and OSA. Altered genioglossus function may contribute to the increased risk of OSA in people with tetraplegia. The precise mechanisms mediating these differences are unknown.
Keywords: sleep-disordered breathing; spinal cord injury; upper airway physiology.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures

Comment in
-
How does spinal cord injury lead to obstructive sleep apnoea?J Physiol. 2018 Jul;596(14):2633. doi: 10.1113/JP276162. Epub 2018 Jun 3. J Physiol. 2018. PMID: 29667192 Free PMC article. No abstract available.
Similar articles
-
Changes in pharyngeal collapsibility and genioglossus reflex responses to negative pressure during the respiratory cycle in obstructive sleep apnoea.J Physiol. 2020 Feb;598(3):567-580. doi: 10.1113/JP278433. Epub 2020 Jan 15. J Physiol. 2020. PMID: 31782971
-
Impaired pharyngeal reflex responses to negative pressure: a novel cause of sleep apnea in multiple sclerosis.J Appl Physiol (1985). 2022 Mar 1;132(3):815-823. doi: 10.1152/japplphysiol.00240.2021. Epub 2022 Jan 20. J Appl Physiol (1985). 2022. PMID: 35050793
-
Regional genioglossus reflex responses to negative pressure pulses in people with obstructive sleep apnea.J Appl Physiol (1985). 2022 Sep 1;133(3):755-765. doi: 10.1152/japplphysiol.00083.2021. Epub 2022 Jun 30. J Appl Physiol (1985). 2022. PMID: 35771222
-
Does episodic hypoxia affect upper airway dilator muscle function? Implications for the pathophysiology of obstructive sleep apnoea.Respir Physiol Neurobiol. 2005 Jul 28;147(2-3):223-34. doi: 10.1016/j.resp.2005.04.001. Respir Physiol Neurobiol. 2005. PMID: 16087148 Review.
-
Phenotypic approaches to obstructive sleep apnoea - New pathways for targeted therapy.Sleep Med Rev. 2018 Feb;37:45-59. doi: 10.1016/j.smrv.2016.12.003. Epub 2016 Dec 18. Sleep Med Rev. 2018. PMID: 28110857 Review.
Cited by
-
A randomised controlled trial of nasal decongestant to treat obstructive sleep apnoea in people with cervical spinal cord injury.Spinal Cord. 2019 Jul;57(7):579-585. doi: 10.1038/s41393-019-0256-6. Epub 2019 Feb 13. Spinal Cord. 2019. PMID: 30760846 Clinical Trial.
-
How does spinal cord injury lead to obstructive sleep apnoea?J Physiol. 2018 Jul;596(14):2633. doi: 10.1113/JP276162. Epub 2018 Jun 3. J Physiol. 2018. PMID: 29667192 Free PMC article. No abstract available.
-
Beyond Usual Care: A Multidisciplinary Approach Towards the Treatment of Obstructive Sleep Apnoea.Front Cardiovasc Med. 2022 Jan 5;8:747495. doi: 10.3389/fcvm.2021.747495. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35071340 Free PMC article. Review.
-
Respiratory-swallow coordination in a rat model of chemoradiation.Head Neck. 2021 Oct;43(10):2954-2966. doi: 10.1002/hed.26782. Epub 2021 Jun 23. Head Neck. 2021. PMID: 34160109 Free PMC article.
-
Impact Of Spinal Cord Injury On Sleep: Current Perspectives.Nat Sci Sleep. 2019 Oct 15;11:219-229. doi: 10.2147/NSS.S197375. eCollection 2019. Nat Sci Sleep. 2019. PMID: 31686935 Free PMC article.
References
-
- Banzett RB, Dempsey JA, O'Donnell DE & Wamboldt MZ (2000). Symptom perception and respiratory sensation in asthma. Am J Resp Crit Care Med 162, 1178–1182. - PubMed
-
- Bensmail D, Salva MAQ, Roche N, Benyahia S, Bohic M, Denys P, Bussel B & Lofaso F (2006). Effect of intrathecal baclofen on sleep and respiratory function in patients with spasticity. Neurology 67, 1432–1436. - PubMed
-
- Berlowitz DJ, Brown DJ, Campbell DA & Pierce RJ (2005). A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch Phys Med Rehabil 86, 1193–1199. - PubMed
-
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM & American Academy of Sleep Medicine (2012). Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8, 597–619. - PMC - PubMed
-
- Boyd JH, Petrof BJ, Hamid Q, Fraser R & Kimoff RJ (2004). Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med 170, 541–546. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

