Strategy to targeting the immune resistance and novel therapy in colorectal cancer
- PMID: 29658188
- PMCID: PMC5943429
- DOI: 10.1002/cam4.1386
Strategy to targeting the immune resistance and novel therapy in colorectal cancer
Abstract
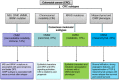
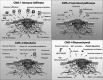
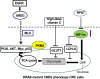
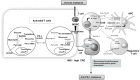
Assessing the CRC subtypes that can predict the outcome of colorectal cancer (CRC) in patients with immunogenicity seems to be a promising strategy to develop new drugs that target the antitumoral immune response. In particular, the disinhibition of the antitumoral T-cell response by immune checkpoint blockade has shown remarkable therapeutic promise for patients with mismatch repair (MMR) deficient CRC. In this review, the authors provide the update of the molecular features and immunogenicity of CRC, discuss the role of possible predictive biomarkers, illustrate the modern immunotherapeutic approaches, and introduce the most relevant ongoing preclinical study and clinical trials such as the use of the combination therapy with immunotherapy. Furthermore, this work is further to understand the complex interactions between the immune surveillance and develop resistance in tumor cells. As expected, if the promise of these developments is fulfilled, it could develop the effective therapeutic strategies and novel combinations to overcome immune resistance and enhance effector responses, which guide clinicians toward a more "personalized" treatment for advanced CRC patients.
Keywords: Colorectal cancer; gene phenotype; immune resistance; immunotherapy; subtypes.
© 2018 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Deficient Mismatch Repair and the Role of Immunotherapy in Metastatic Colorectal Cancer.Curr Treat Options Oncol. 2016 Aug;17(8):41. doi: 10.1007/s11864-016-0414-4. Curr Treat Options Oncol. 2016. PMID: 27315067 Review.
-
Genomics and emerging biomarkers for immunotherapy of colorectal cancer.Semin Cancer Biol. 2018 Oct;52(Pt 2):189-197. doi: 10.1016/j.semcancer.2018.02.010. Epub 2018 Mar 1. Semin Cancer Biol. 2018. PMID: 29501787 Review.
-
Clinical Development of Immunotherapy for Deficient Mismatch Repair Colorectal Cancer.Clin Colorectal Cancer. 2020 Jun;19(2):73-81. doi: 10.1016/j.clcc.2020.02.002. Epub 2020 Feb 10. Clin Colorectal Cancer. 2020. PMID: 32173280 Review.
-
Immunotherapy for colorectal cancer: where are we heading?Expert Opin Biol Ther. 2017 Jun;17(6):709-721. doi: 10.1080/14712598.2017.1315405. Epub 2017 Apr 13. Expert Opin Biol Ther. 2017. PMID: 28375039 Review.
-
Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside.Biochim Biophys Acta Rev Cancer. 2020 Dec;1874(2):188447. doi: 10.1016/j.bbcan.2020.188447. Epub 2020 Oct 6. Biochim Biophys Acta Rev Cancer. 2020. PMID: 33035640 Free PMC article. Review.
Cited by
-
The Anti-Tumor Effect and Mechanism of Triterpenoids in Rhus chinensis Mill. on Reversing Effector CD8+ T-cells Dysfunction by Targeting Glycolysis Pathways in Colorectal Cancer.Integr Cancer Ther. 2021 Jan-Dec;20:15347354211017219. doi: 10.1177/15347354211017219. Integr Cancer Ther. 2021. PMID: 34014135 Free PMC article.
-
A prognostic model for colorectal cancer based on CEA and a 48-multiplex serum biomarker panel.Sci Rep. 2021 Feb 22;11(1):4287. doi: 10.1038/s41598-020-80785-1. Sci Rep. 2021. PMID: 33619304 Free PMC article.
-
Immune Tolerance vs. Immune Resistance: The Interaction Between Host and Pathogens in Infectious Diseases.Front Vet Sci. 2022 Mar 29;9:827407. doi: 10.3389/fvets.2022.827407. eCollection 2022. Front Vet Sci. 2022. PMID: 35425833 Free PMC article.
-
Targeting HDAC2-Mediated Immune Regulation to Overcome Therapeutic Resistance in Mutant Colorectal Cancer.Cancers (Basel). 2023 Mar 24;15(7):1960. doi: 10.3390/cancers15071960. Cancers (Basel). 2023. PMID: 37046620 Free PMC article.
-
Effect of colorectal cancer stem cells on the development and metastasis of colorectal cancer.World J Gastrointest Oncol. 2024 Nov 15;16(11):4354-4368. doi: 10.4251/wjgo.v16.i11.4354. World J Gastrointest Oncol. 2024. PMID: 39554751 Free PMC article. Review.
References
-
- Basile, D. , Garattini S. K., Bonotto M., Ongaro E., Casagrande M., Cattaneo M., et al. 2017. Immunotherapy for colorectal cancer: where are we heading? Expert Opin. Biol. Ther. 17:709–721. - PubMed
-
- Mao, H. , Pan F., Wu Z., Wang Z., Zhou Y., Zhang P., et al. 2017. Colorectal tumors are enriched with regulatory plasmablasts with capacity in suppressing T cell inflammation. Int. Immunopharmacol. 49:95–101. - PubMed
-
- Pages, F. , Berger A., Camus M., Sanchez‐Cabo F., Costes A., Molidor R., et al. 2005. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. Med. 353:2654–2666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

