Open-Source Potentiostat for Wireless Electrochemical Detection with Smartphones
- PMID: 29658268
- PMCID: PMC5997382
- DOI: 10.1021/acs.analchem.8b00850
Open-Source Potentiostat for Wireless Electrochemical Detection with Smartphones
Abstract
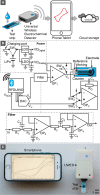
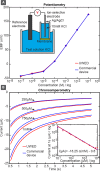
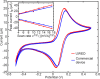
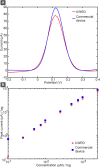
This paper describes the design and characterization of an open-source "universal wireless electrochemical detector" (UWED). This detector interfaces with a smartphone (or a tablet) using "Bluetooth Low Energy" protocol; the smartphone provides (i) a user interface for receiving the experimental parameters from the user and visualizing the result in real time, and (ii) a proxy for storing, processing, and transmitting the data and experimental protocols. This approach simplifies the design, and decreases both the size and the cost of the hardware; it also makes the UWED adaptable to different types of analyses by simple modification of the software. The UWED can perform the most common electroanalytical techniques of potentiometry, chronoamperometry, cyclic voltammetry, and square wave voltammetry, with results closely comparable to benchtop commercial potentiostats. Although the operating ranges of electrical current and voltage of the UWED (±1.5 V, ±180 μA) are more limited than most benchtop commercial potentiostats, its functional range is sufficient for most electrochemical analyses in aqueous solutions. Because the UWED is simple, small in size, assembled from inexpensive components, and completely wireless, it offers new opportunities for the development of affordable diagnostics, sensors, and wearable devices.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

