Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles
- PMID: 29658707
- PMCID: PMC6061779
- DOI: 10.1021/acs.chemrev.7b00776
Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles
Abstract
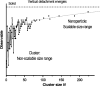
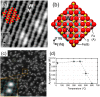
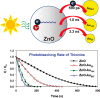
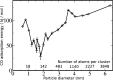
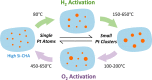
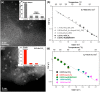
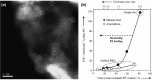
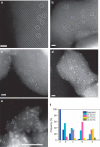
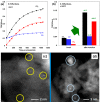
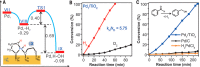
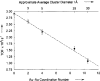
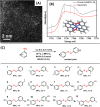
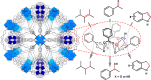
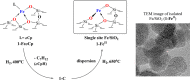
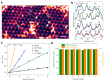
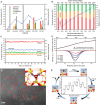
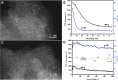
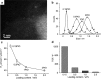
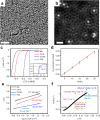
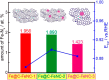
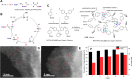
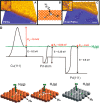
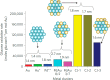
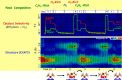
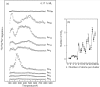
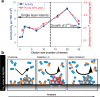
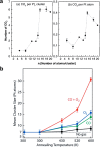
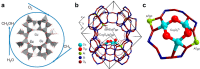
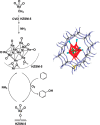
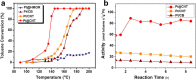
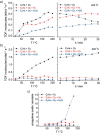
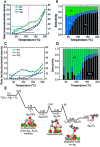
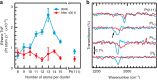
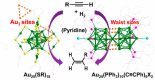
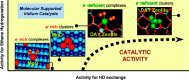
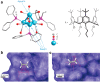
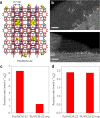
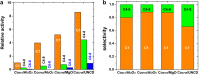
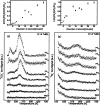
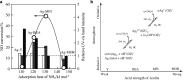
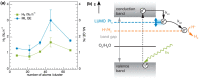
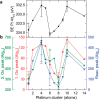
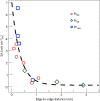
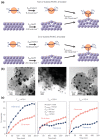
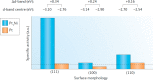
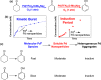
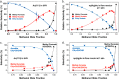
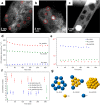
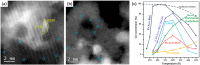
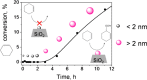
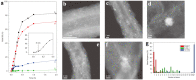
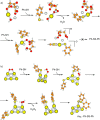
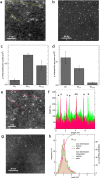
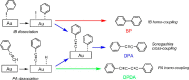
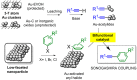
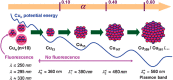
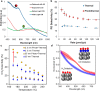
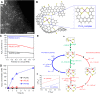
Metal species with different size (single atoms, nanoclusters, and nanoparticles) show different catalytic behavior for various heterogeneous catalytic reactions. It has been shown in the literature that many factors including the particle size, shape, chemical composition, metal-support interaction, and metal-reactant/solvent interaction can have significant influences on the catalytic properties of metal catalysts. The recent developments of well-controlled synthesis methodologies and advanced characterization tools allow one to correlate the relationships at the molecular level. In this Review, the electronic and geometric structures of single atoms, nanoclusters, and nanoparticles will be discussed. Furthermore, we will summarize the catalytic applications of single atoms, nanoclusters, and nanoparticles for different types of reactions, including CO oxidation, selective oxidation, selective hydrogenation, organic reactions, electrocatalytic, and photocatalytic reactions. We will compare the results obtained from different systems and try to give a picture on how different types of metal species work in different reactions and give perspectives on the future directions toward better understanding of the catalytic behavior of different metal entities (single atoms, nanoclusters, and nanoparticles) in a unifying manner.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

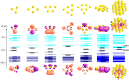










































References
-
- Boudart M. Catalysis by Supported Metals. Adv. Catal. 1969, 20, 153–166. 10.1016/S0360-0564(08)60271-0. - DOI
-
- Boudart M. Heterogeneous Catalysis by Metals. J. Mol. Catal. 1985, 30, 27–38. 10.1016/0304-5102(85)80014-6. - DOI
-
- Ertl G., Knözinger H., Weitkamp J., Eds. Handbook of Heterogeneous Catalysis; Wiley-VCH: New York, 1997.
-
- Čejka J., Corma A., Zones S., Eds. Zeolites and Catalysis: Synthesis, Reactions and Applications; Wiley-VCH: New York, 2010.
-
- Čejka J., Morris R. E., Nachtigall P., Eds. Zeolites in Catalysis: Properties and Applications; Royal Society of Chemistry: UK, 2017.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

