Scandium and terbium radionuclides for radiotheranostics: current state of development towards clinical application
- PMID: 29658792
- PMCID: PMC6475947
- DOI: 10.1259/bjr.20180074
Scandium and terbium radionuclides for radiotheranostics: current state of development towards clinical application
Abstract
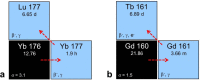
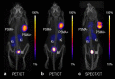
Currently, different radiometals are in use for imaging and therapy in nuclear medicine: 68Ga and 111In are examples of nuclides for positron emission tomography (PET) and single photon emission computed tomography (SPECT), respectively, while 177Lu and 225Ac are used for β-- and α-radionuclide therapy. The application of diagnostic and therapeutic radionuclides of the same element (radioisotopes) would utilize chemically-identical radiopharmaceuticals for imaging and subsequent treatment, thereby enabling the radiotheranostic concept. There are two elements which are of particular interest in this regard: Scandium and Terbium. Scandium presents three radioisotopes for theranostic application. 43Sc (T1/2 = 3.9 h) and 44Sc (T1/2 = 4.0 h) can both be used for PET, while 47Sc (T1/2 = 3.35 d) is the therapeutic match-also suitable for SPECT. Currently, 44Sc is most advanced in terms of production, as well as with pre-clinical investigations, and has already been employed in proof-of-concept studies in patients. Even though the production of 43Sc may be more challenging, it would be advantageous due to the absence of high-energetic γ-ray emission. The development of 47Sc is still in its infancy, however, its therapeutic potential has been demonstrated preclinically. Terbium is unique in that it represents four medically-interesting radioisotopes. 155Tb (T1/2 = 5.32 d) and 152Tb (T1/2 = 17.5 h) can be used for SPECT and PET, respectively. Both radioisotopes were produced and tested preclinically. 152Tb has been the first Tb isotope that was tested (as 152Tb-DOTATOC) in a patient. Both radionuclides may be of interest for dosimetry purposes prior to the application of radiolanthanide therapy. The decay properties of 161Tb (T1/2 = 6.89 d) are similar to 177Lu, but the coemission of Auger electrons make it attractive for a combined β-/Auger electron therapy, which was shown to be effective in preclinical experiments. 149Tb (T1/2 = 4.1 h) has been proposed for targeted α-therapy with the possibility of PET imaging. In terms of production, 161Tb and 155Tb are most promising to be made available at the large quantities suitable for future clinical translation. This review article is dedicated to the production routes, the methods of separating the radioisotopes from the target material, preclinical investigations and clinical proof-of-concept studies of Sc and Tb radionuclides. The availability, challenges of production and first (pre)clinical application, as well as the potential of these novel radionuclides for future application in nuclear medicine, are discussed.
Figures
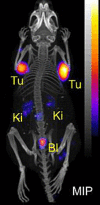
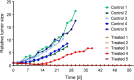
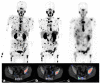
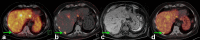
References
-
- Hertz B, Schuller K, Saul Hertz M. Saul Hertz, MD (1905–1950): A pioneer in the use of radioactive Iodine. Endocr Pract 2010; 16: 713–5. doi: 10.4158/EP10065.CO - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

