Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden
- PMID: 29658845
- PMCID: PMC7193684
- DOI: 10.1056/NEJMoa1801946
Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden
Abstract
Background: Nivolumab plus ipilimumab showed promising efficacy for the treatment of non-small-cell lung cancer (NSCLC) in a phase 1 trial, and tumor mutational burden has emerged as a potential biomarker of benefit. In this part of an open-label, multipart, phase 3 trial, we examined progression-free survival with nivolumab plus ipilimumab versus chemotherapy among patients with a high tumor mutational burden (≥10 mutations per megabase).
Methods: We enrolled patients with stage IV or recurrent NSCLC that was not previously treated with chemotherapy. Those with a level of tumor programmed death ligand 1 (PD-L1) expression of at least 1% were randomly assigned, in a 1:1:1 ratio, to receive nivolumab plus ipilimumab, nivolumab monotherapy, or chemotherapy; those with a tumor PD-L1 expression level of less than 1% were randomly assigned, in a 1:1:1 ratio, to receive nivolumab plus ipilimumab, nivolumab plus chemotherapy, or chemotherapy. Tumor mutational burden was determined by the FoundationOne CDx assay.
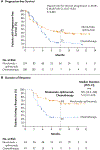
Results: Progression-free survival among patients with a high tumor mutational burden was significantly longer with nivolumab plus ipilimumab than with chemotherapy. The 1-year progression-free survival rate was 42.6% with nivolumab plus ipilimumab versus 13.2% with chemotherapy, and the median progression-free survival was 7.2 months (95% confidence interval [CI], 5.5 to 13.2) versus 5.5 months (95% CI, 4.4 to 5.8) (hazard ratio for disease progression or death, 0.58; 97.5% CI, 0.41 to 0.81; P<0.001). The objective response rate was 45.3% with nivolumab plus ipilimumab and 26.9% with chemotherapy. The benefit of nivolumab plus ipilimumab over chemotherapy was broadly consistent within subgroups, including patients with a PD-L1 expression level of at least 1% and those with a level of less than 1%. The rate of grade 3 or 4 treatment-related adverse events was 31.2% with nivolumab plus ipilimumab and 36.1% with chemotherapy. ical; CheckMate 227 ClinicalTrials.gov number, NCT02477826 .).
Conclusions: Progression-free survival was significantly longer with first-line nivolumab plus ipilimumab than with chemotherapy among patients with NSCLC and a high tumor mutational burden, irrespective of PD-L1 expression level. The results validate the benefit of nivolumab plus ipilimumab in NSCLC and the role of tumor mutational burden as a biomarker for patient selection. (Funded by Bristol-Myers Squibb and Ono Pharmaceut
Figures


Comment in
-
High TMB Predicts Immunotherapy Benefit.Cancer Discov. 2018 Jun;8(6):668. doi: 10.1158/2159-8290.CD-NB2018-048. Epub 2018 Apr 16. Cancer Discov. 2018. PMID: 29661758
-
Nivolumab-ipilimumab - exploiting the mutation burden of NSCLCs.Nat Rev Clin Oncol. 2018 Jul;15(7):403. doi: 10.1038/s41571-018-0034-y. Nat Rev Clin Oncol. 2018. PMID: 29703915 No abstract available.
-
New windows open for immunotherapy in lung cancer.Nature. 2018 Jun;558(7710):376-377. doi: 10.1038/d41586-018-05312-9. Nature. 2018. PMID: 29907821 No abstract available.
-
Immune Checkpoint Blockade across the Cancer Care Continuum.Immunity. 2018 Jun 19;48(6):1077-1080. doi: 10.1016/j.immuni.2018.06.003. Immunity. 2018. PMID: 29924973
-
Frontline immunotherapy for NSCLC: alone or not alone?Nat Rev Clin Oncol. 2018 Oct;15(10):593-594. doi: 10.1038/s41571-018-0070-7. Nat Rev Clin Oncol. 2018. PMID: 29993034 No abstract available.
-
Radiotherapy, tumor mutational burden, and immune checkpoint inhibitors: time to do the math.Strahlenther Onkol. 2018 Oct;194(10):873-875. doi: 10.1007/s00066-018-1341-z. Epub 2018 Jul 20. Strahlenther Onkol. 2018. PMID: 30030581 No abstract available.
-
Predictive biomarkers for immune checkpoint inhibitor therapy: we need to keep searching.J Thorac Dis. 2018 Jul;10(Suppl 18):S2195-S2197. doi: 10.21037/jtd.2018.06.144. J Thorac Dis. 2018. PMID: 30123559 Free PMC article. No abstract available.
-
Lung Cancer with a High Tumor Mutational Burden.N Engl J Med. 2018 Sep 13;379(11):1093. doi: 10.1056/NEJMc1808566. N Engl J Med. 2018. PMID: 30211492 No abstract available.
-
Tumor mutation burden in lung cancer: a new predictive biomarker for immunotherapy or too soon to tell?J Thorac Dis. 2018 Nov;10(Suppl 33):S3994-S3998. doi: 10.21037/jtd.2018.09.35. J Thorac Dis. 2018. PMID: 30631537 Free PMC article. No abstract available.
-
Immunotherapy in Non-Small Cell Lung Cancer. Which Patients and at Which Stage?Am J Respir Crit Care Med. 2019 May 15;199(10):1277-1279. doi: 10.1164/rccm.201810-1930RR. Am J Respir Crit Care Med. 2019. PMID: 30860861 No abstract available.
References
-
- Hanna N, Johnson D, Temin S, Masters G. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update summary. J Oncol Pract 2017; 13: 832–7. - PubMed
-
- Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15: 504–35. - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016; 375: 1823–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
