Neoadjuvant PD-1 Blockade in Resectable Lung Cancer
- PMID: 29658848
- PMCID: PMC6223617
- DOI: 10.1056/NEJMoa1716078
Neoadjuvant PD-1 Blockade in Resectable Lung Cancer
Erratum in
-
Neoadjuvant PD-1 Blockade in Resectable Lung Cancer; Nivolumab and Ipilimumab in Advanced Melanoma; Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma; Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy; Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma; Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma; Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma; Rapid Eradication of a Bulky Melanoma Mass with One Dose of Immunotherapy; Genetic Basis for Clinical Response to CTLA-4 Blockade; Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma; Nivolumab plus Ipilimumab in Advanced Melanoma; Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma; Hepatotoxicity with Combination of Vemurafenib and Ipilimumab.N Engl J Med. 2018 Nov 29;379(22):2185. doi: 10.1056/NEJMx180040. Epub 2018 Nov 9. N Engl J Med. 2018. PMID: 31442371 No abstract available.
Abstract
Background: Antibodies that block programmed death 1 (PD-1) protein improve survival in patients with advanced non-small-cell lung cancer (NSCLC) but have not been tested in resectable NSCLC, a condition in which little progress has been made during the past decade.
Methods: In this pilot study, we administered two preoperative doses of PD-1 inhibitor nivolumab in adults with untreated, surgically resectable early (stage I, II, or IIIA) NSCLC. Nivolumab (at a dose of 3 mg per kilogram of body weight) was administered intravenously every 2 weeks, with surgery planned approximately 4 weeks after the first dose. The primary end points of the study were safety and feasibility. We also evaluated the tumor pathological response, expression of programmed death ligand 1 (PD-L1), mutational burden, and mutation-associated, neoantigen-specific T-cell responses.
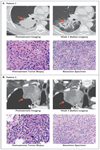
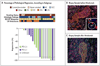
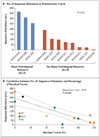
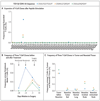
Results: Neoadjuvant nivolumab had an acceptable side-effect profile and was not associated with delays in surgery. Of the 21 tumors that were removed, 20 were completely resected. A major pathological response occurred in 9 of 20 resected tumors (45%). Responses occurred in both PD-L1-positive and PD-L1-negative tumors. There was a significant correlation between the pathological response and the pretreatment tumor mutational burden. The number of T-cell clones that were found in both the tumor and peripheral blood increased systemically after PD-1 blockade in eight of nine patients who were evaluated. Mutation-associated, neoantigen-specific T-cell clones from a primary tumor with a complete response on pathological assessment rapidly expanded in peripheral blood at 2 to 4 weeks after treatment; some of these clones were not detected before the administration of nivolumab.
Conclusions: Neoadjuvant nivolumab was associated with few side effects, did not delay surgery, and induced a major pathological response in 45% of resected tumors. The tumor mutational burden was predictive of the pathological response to PD-1 blockade. Treatment induced expansion of mutation-associated, neoantigen-specific T-cell clones in peripheral blood. (Funded by Cancer Research Institute-Stand Up 2 Cancer and others; ClinicalTrials.gov number, NCT02259621 .).
Figures
Comment in
-
Neoadjuvant Nivolumab Checks Lung Cancer.Cancer Discov. 2018 Jun;8(6):667-668. doi: 10.1158/2159-8290.CD-NB2018-049. Epub 2018 Apr 16. Cancer Discov. 2018. PMID: 29661759
-
Neoadjuvant PD-1 blockade in NSCLC.Nat Rev Clin Oncol. 2018 Jul;15(7):404. doi: 10.1038/s41571-018-0032-0. Nat Rev Clin Oncol. 2018. PMID: 29700379 No abstract available.
-
Fast Forward - Neoadjuvant Cancer Immunotherapy.N Engl J Med. 2018 May 24;378(21):2034-2035. doi: 10.1056/NEJMe1803923. N Engl J Med. 2018. PMID: 29791816 No abstract available.
-
Mediastinal lymph node clearance and anti-PD-1 induction in resected NSCLC.Ann Oncol. 2018 Aug 1;29(8):1879. doi: 10.1093/annonc/mdy200. Ann Oncol. 2018. PMID: 29873686 No abstract available.
-
New windows open for immunotherapy in lung cancer.Nature. 2018 Jun;558(7710):376-377. doi: 10.1038/d41586-018-05312-9. Nature. 2018. PMID: 29907821 No abstract available.
-
Immune Checkpoint Blockade across the Cancer Care Continuum.Immunity. 2018 Jun 19;48(6):1077-1080. doi: 10.1016/j.immuni.2018.06.003. Immunity. 2018. PMID: 29924973
-
Does oncology research need to slow down a little bit?J Oncol Pharm Pract. 2019 Jan;25(1):247-249. doi: 10.1177/1078155218785238. Epub 2018 Jun 27. J Oncol Pharm Pract. 2019. PMID: 29945533 No abstract available.
-
Neoadjuvant PD-1 Blockade in Resectable Lung Cancer.N Engl J Med. 2018 Aug 30;379(9):e14. doi: 10.1056/NEJMc1808251. N Engl J Med. 2018. PMID: 30179394 No abstract available.
-
Neoadjuvant PD-1 Blockade in Resectable Lung Cancer.N Engl J Med. 2018 Aug 30;379(9):e14. doi: 10.1056/NEJMc1808251. N Engl J Med. 2018. PMID: 30179396 No abstract available.
-
Immunotherapy and molecular role of T-cell in PD-1 antibody treated resectable lung cancer patients.J Thorac Dis. 2018 Aug;10(8):4682-4685. doi: 10.21037/jtd.2018.07.66. J Thorac Dis. 2018. PMID: 30233838 Free PMC article. No abstract available.
-
Neoadjuvant PD-1 blockade in lung cancer: we're not in Kansas anymore.J Thorac Dis. 2018 Aug;10(8):4686-4688. doi: 10.21037/jtd.2018.07.98. J Thorac Dis. 2018. PMID: 30233839 Free PMC article. No abstract available.
-
Neoadjuvant immunotherapy for non-small cell lung cancer: can early intervention result in durable clinical benefit?J Thorac Dis. 2018 Sep;10(Suppl 26):S3203-S3206. doi: 10.21037/jtd.2018.08.39. J Thorac Dis. 2018. PMID: 30370113 Free PMC article. No abstract available.
-
Early stage resectable non-small cell lung cancer: is neoadjuvant immunotherapy the right way forward?J Thorac Dis. 2018 Nov;10(Suppl 33):S3890-S3894. doi: 10.21037/jtd.2018.10.22. J Thorac Dis. 2018. PMID: 30631508 Free PMC article. No abstract available.
-
Immunotherapy in Non-Small Cell Lung Cancer. Which Patients and at Which Stage?Am J Respir Crit Care Med. 2019 May 15;199(10):1277-1279. doi: 10.1164/rccm.201810-1930RR. Am J Respir Crit Care Med. 2019. PMID: 30860861 No abstract available.
References
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016;375: 1823–33. - PubMed
-
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 2017;377:1919–29. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
