Cost-Effectiveness Analyses of the 21-Gene Assay in Breast Cancer: Systematic Review and Critical Appraisal
- PMID: 29659329
- PMCID: PMC5978470
- DOI: 10.1200/JCO.2017.76.5941
Cost-Effectiveness Analyses of the 21-Gene Assay in Breast Cancer: Systematic Review and Critical Appraisal
Abstract
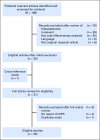
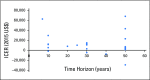
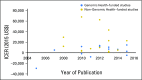
Purpose Prior studies examining cost effectiveness of the 21-gene assay (Oncotype DX [ODX]) for women with hormone receptor-positive, early-stage breast cancer have yielded disparate results. We aimed to explore why these analyses may have yielded different conclusions. Methods We conducted a systematic literature review of cost-effectiveness analyses (CEAs) of ODX. We examined the extent to which the structure of CEA modeling, the assumptions of the models, and the selection of input parameters influenced cost-effectiveness estimates. We also explored the prevalence of industry funding and whether industry funding was associated with study designs favoring ODX. Results We identified 27 analyses, 15 of which received industry funding. In 18 studies, the clinical characteristics (eg, tumor size and grade) commonly used to make chemotherapy decisions were not incorporated into simulation modeling; thus, these studies would favor ODX being cost effective and might not reflect clinical practice. Most studies ignored the heterogeneous effect of ODX on chemotherapy use; only five studies assumed that ODX would increase chemotherapy use for clinically low-risk patients but decrease chemotherapy use for clinically high-risk patients. No study used population-based joint distributions of ODX recurrence score and tumor characteristics, and 12 studies inappropriately assumed that chemotherapy would increase distant recurrence for the low recurrence score group; both approaches overestimated the benefits of ODX. Industry-funded studies tended to favor ODX; all five studies that reported ODX as being cost saving were industry funded. In contrast, two studies that reported an incremental cost-effectiveness ratio > $50,000 per quality-adjusted life-year were not funded by industry. Conclusion Although a majority of published analyses indicated that ODX is cost effective, they incorporated study designs that can increase the risk of bias.
Figures


References
-
- Marchionni L, Wilson RF, Wolff AC, et al. : Systematic review: gene expression profiling assays in early-stage breast cancer. Ann Intern Med 148:358-369, 2008 - PubMed
-
- Paik S, Shak S, Tang G, et al. : A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817-2826, 2004 - PubMed
-
- Hayes DF: Targeting adjuvant chemotherapy: A good idea that needs to be proven! J Clin Oncol 30:1264-1267, 2012 - PubMed
-
- Hassett MJ, O’Malley AJ, Pakes JR, et al. : Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst 98:1108-1117, 2006 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

