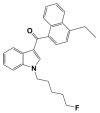
In Vitro Inhibitory Effects of Synthetic Cannabinoid EAM-2201 on Cytochrome P450 and UDP-Glucuronosyltransferase Enzyme Activities in Human Liver Microsomes
- PMID: 29659506
- PMCID: PMC6017357
- DOI: 10.3390/molecules23040920
In Vitro Inhibitory Effects of Synthetic Cannabinoid EAM-2201 on Cytochrome P450 and UDP-Glucuronosyltransferase Enzyme Activities in Human Liver Microsomes
Abstract
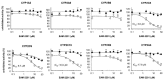
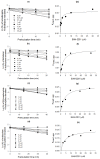
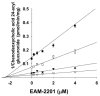
EAM-2201, a synthetic cannabinoid, is a potent agonist of the cannabinoid receptors that is widely abused as an illicit recreational drug in combination with other drugs. To evaluate the potential of EAM-2201 as a perpetrator of drug−drug interactions, the inhibitory effects of EAM-2201 on major drug-metabolizing enzymes, cytochrome P450s (CYPs) and uridine 5′-diphospho-glucuronosyltransferases (UGTs) were evaluated in pooled human liver microsomes using liquid chromatography−tandem mass spectrometry (LC-MS/MS). EAM-2201 at doses up to 50 µM negligibly inhibited the activities of eight major human CYPs (1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4) and five UGTs (1A1, 1A4, 1A6, 1A9 and 2B7) in human liver microsomes. EAM-2201 exhibited time-dependent inhibition of CYP2C8-catalyzed amodiaquine N-deethylation, CYP2C9-catalyzed diclofenac 4′-hydroxylation, CYP2C19-catalyzed [S]-mephenytoin 4′-hydroxylation and CYP3A4-catalyzed midazolam 1′-hydroxylation with Ki values of 0.54 µM (kinact: 0.0633 min−1), 3.0 µM (kinact: 0.0462 min−1), 3.8 µM (kinact: 0.0264 min−1) and 4.1 µM (kinact: 0.0250 min−1), respectively and competitively inhibited UGT1A3-catalyzed chenodeoxycholic acid 24-acyl-glucuronidation, with a Ki value of 2.4 µM. Based on these in vitro results, we conclude that EAM-2201 has the potential to trigger in vivo pharmacokinetic drug interactions when co-administered with substrates of CYP2C8, CYP2C9, CYP2C19, CYP3A4 and UGT1A3.
Keywords: EAM-2201; UDP-glucuronosyltransferase; cytochrome P450; drug–drug interaction; human liver microsomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

