Effects of mTOR-Is on malignancy and survival following renal transplantation: A systematic review and meta-analysis of randomized trials with a minimum follow-up of 24 months
- PMID: 29659588
- PMCID: PMC5901925
- DOI: 10.1371/journal.pone.0194975
Effects of mTOR-Is on malignancy and survival following renal transplantation: A systematic review and meta-analysis of randomized trials with a minimum follow-up of 24 months
Abstract
Background: mTOR-Is positively influence the occurrence and course of certain tumors after solid organ transplantation. The effect of mTOR-Is on the overall incidence of tumors irrespective of their origin is not entirely clear. Furthermore, conflicting data have been shown on mortality under mTOR-Is.
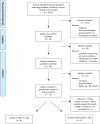
Methods: The current literature was searched for prospective randomized controlled renal transplantation trials. There were 1415 trials screened of which 13 could be included (pts. = 5924). A minimum follow-up of 24 months was mandatory for inclusion. Incidence of malignancies and patient survival was assessed in meta-analyses.
Results: The average follow-up of all trials was 40.6 months. Malignancy was significantly reduced under mTOR-Is compared to CNIs (RR 0.70, CI 0.49-0.99, p = 0.046). This effect remained stable when combined with CNIs (RR 0.58, CI 0.34-1.00, p = 0.05). When NMSCs were excluded the risk for malignancy remained significantly reduced under mTOR-I therapy (mono and combi) (RR 0.43, CI 0.24-0.77, p = 0.0046). Graft survival was minimally decreased under mTOR-Is (RR 0.99, CI 0.98-1.00, p = 0.054). This effect was abrogated when mTOR-Is were combined with CNIs (RR 0.99, CI 0.97-1.02, p = 0.50). Patient survival was not different (RR 1.00, CI 0.99-1.01, p = 0.54).
Conclusions: Posttransplant patients have a lower incidence of malignancy when treated with an mTOR-I no matter if it is used in combination with CNIs or not. This beneficial effect remains significant even when NMSCs are excluded. With currently used mTOR-I-based regimen patient and graft survival is not different compared to CNI therapies.
Conflict of interest statement
Figures





References
-
- Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21(1):153–61. Epub 2009/10/31. doi: 10.1681/ASN.2009040412 . - DOI - PMC - PubMed
-
- Opelz G, Dohler B. Association of HLA mismatch with death with a functioning graft after kidney transplantation: a collaborative transplant study report. Am J Transplant. 2012;12(11):3031–8. Epub 2012/08/21. doi: 10.1111/j.1600-6143.2012.04226.x . - DOI - PubMed
-
- Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, et al. The janus face of immunosuppression—de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney Int. 2007;71(12):1271–8. Epub 2007/03/03. doi: 10.1038/sj.ki.5002154 . - DOI - PubMed
-
- Domhan S, Zeier M, Abdollahi A. Immunosuppressive therapy and post-transplant malignancy. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2009;24(4):1097–103. Epub 2008/11/04. doi: 10.1093/ndt/gfn605 . - DOI - PubMed
-
- Penn I. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transpl. 1998:147–58. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

