Reconstruction of a 10-mm-long median nerve gap in an ischemic environment using autologous conduits with different patterns of blood supply: A comparative study in the rat
- PMID: 29659600
- PMCID: PMC5902043
- DOI: 10.1371/journal.pone.0195692
Reconstruction of a 10-mm-long median nerve gap in an ischemic environment using autologous conduits with different patterns of blood supply: A comparative study in the rat
Abstract
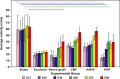
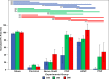
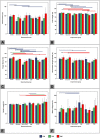
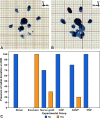
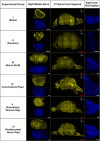
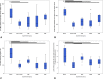
The aim of this study was to evaluate in the Wistar rat the efficacy of various autologous nerve conduits with various forms of blood supply in reconstructing a 10-mm-long gap in the median nerve (MN) under conditions of local ischemia. A 10-mm-long median nerve defect was created in the right arm. A loose silicone tube was placed around the nerve gap zone, in order to simulate a local ischemic environment. Rats were divided in the following experimental groups (each with 20 rats): the nerve Graft (NG) group, in which the excised MN segment was reattached; the conventional nerve flap (CNF) and the arterialized neurovenous flap (ANVF) groups in which the gap was bridged with homonymous median nerve flaps; the prefabricated nerve flap (PNF) group in which the gap was reconstructed with a fabricated flap created by leaving an arteriovenous fistula in contact with the sciatic nerve for 5 weeks; and the two control groups, Sham and Excision groups. In the latter group, the proximal stump of the MN nerve was ligated and no repair was performed. The rats were followed for 100 days. During this time, they did physiotherapy. Functional, electroneuromyographic and histological studies were performed. The CNF and ANVF groups presented better results than the NG group in the following assessments: grasping test, nociception, motor stimulation threshold, muscle weight, and histomorphometric evaluation. Radial deviation of the operated forepaw was more common in rats that presented worse results in the other outcome variables. Overall, CNFs and ANVFs produced a faster and more complete recovery than NGs in the reconstruction of a 10-mm-long median nerve gap in an ischemic environment in the Wistar rat. Although, results obtained with CNFs were in most cases were better than ANVFs, these differences were not statistically significant for most of the outcome variables.
Conflict of interest statement
Figures







Similar articles
-
Reconstruction of a long defect of the ulnar artery and nerve with an arterialized neurovenous free flap in a teenager: A case report and literature review.Microsurgery. 2018 Feb;38(2):209-217. doi: 10.1002/micr.30265. Epub 2017 Nov 2. Microsurgery. 2018. PMID: 29094390 Free PMC article. Review.
-
Better nerve regeneration with distally based fascicular turnover flap than with conventional autologous nerve graft in a rat sciatic nerve defect model.J Plast Reconstr Aesthet Surg. 2020 Feb;73(2):214-221. doi: 10.1016/j.bjps.2019.09.031. Epub 2019 Oct 1. J Plast Reconstr Aesthet Surg. 2020. PMID: 31690543
-
Consequences of oversizing: nerve-to-nerve tube diameter mismatch.J Bone Joint Surg Am. 2014 Sep 3;96(17):1461-7. doi: 10.2106/JBJS.M.01420. J Bone Joint Surg Am. 2014. PMID: 25187585
-
Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: An experimental study.Microsurgery. 2016 Nov;36(8):664-671. doi: 10.1002/micr.22418. Epub 2015 Apr 22. Microsurgery. 2016. PMID: 25899554
-
Functional recovery and mechanisms in end-to-side nerve repair in rats.Acta Neurochir Suppl. 2007;100:93-5. doi: 10.1007/978-3-211-72958-8_20. Acta Neurochir Suppl. 2007. PMID: 17985554 Review.
Cited by
-
Evaluation of Functional Recovery in Rats After Median Nerve Resection and Autograft Repair Using Computerized Gait Analysis.Front Neurosci. 2021 Jan 21;14:593545. doi: 10.3389/fnins.2020.593545. eCollection 2020. Front Neurosci. 2021. PMID: 33551723 Free PMC article.
-
Multimodular Bio-Inspired Organized Structures Guiding Long-Distance Axonal Regeneration.Biomedicines. 2022 Sep 8;10(9):2228. doi: 10.3390/biomedicines10092228. Biomedicines. 2022. PMID: 36140328 Free PMC article.
-
Chitin scaffold combined with autologous small nerve repairs sciatic nerve defects.Neural Regen Res. 2022 May;17(5):1106-1114. doi: 10.4103/1673-5374.324859. Neural Regen Res. 2022. PMID: 34558539 Free PMC article.
-
The Median Nerve Injury Model in Pre-clinical Research - A Critical Review on Benefits and Limitations.Front Cell Neurosci. 2019 Jun 28;13:288. doi: 10.3389/fncel.2019.00288. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31316355 Free PMC article. Review.
-
Review of Piezoelectrical Materials Potentially Useful for Peripheral Nerve Repair.Biomedicines. 2023 Dec 1;11(12):3195. doi: 10.3390/biomedicines11123195. Biomedicines. 2023. PMID: 38137416 Free PMC article. Review.
References
-
- Desouches C, Alluin O, Mutaftschiev N, Dousset E, Magalon G, Boucraut J, et al. [Peripheral nerve repair: 30 centuries of scientific research]. Revue neurologique. 2005;161(11):1045–59. Epub 2005/11/17. . - PubMed
-
- Boyd KU, Fox IK. Nerve repair and grafting In: Mackinnon SE, editor. Nerve surgery. 1. First ed New York: Thieme; 2015. p. 75–100.
-
- Wood MJ, Johnson PJ, Myckatyn TM. Anatomy and physiology for the peripheral nerve surgeon In: Mackinnon SE, Yee A, editors. Nerve Surgery. 1. First ed New York: Thieme; 2015. p. 1–40.
-
- Missios S, Bekelis K, Roberts DW. Neurosurgery in the Byzantine Empire: the contributions of Paul of Aegina (625–690 AD). Journal of neurosurgery. 2014;120(1):244–9. Epub 2013/09/17. doi: 10.3171/2013.8.JNS13550 . - DOI - PubMed
-
- Philipeaux J, Vulpian A. Note sur des essais de greffe d'un troncon du nerf lingual entre les deux bouts du nerf hypoglosse, apres excision d'un segment de ce dernier nerf. Arch Physiol Norm Pathol. 1870;3:618–20.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

