Depletion of sucrose induces changes in the tip growth mechanism of tobacco pollen tubes
- PMID: 29659664
- PMCID: PMC6025209
- DOI: 10.1093/aob/mcy043
Depletion of sucrose induces changes in the tip growth mechanism of tobacco pollen tubes
Abstract
Background and aims: Pollen tubes are rapidly growing, photosynthetically inactive cells that need high rates of energy to support growth. Energy can derive from internal and external storage sources. The lack of carbon sources can cause various problems during pollen tube growth, which in turn could affect the reproduction of plants.
Methods: We analysed the effects of energy deficiency on the development of Nicotiana tabacum pollen tubes by replacing sucrose with glycerol in the growth medium. We focused on cell growth and related processes, such as metabolite composition and cell wall synthesis.
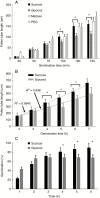
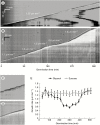
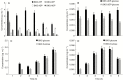
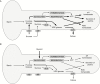
Key results: We found that the lack of sucrose affects pollen germination and pollen tube length during a specific growth period. Both sugar metabolism and ATP concentration were affected by sucrose shortage when pollen tubes were grown in glycerol-based media; this was related to decreases in the concentrations of glucose, fructose and UDP-glucose. The intracellular pH and ROS levels also showed a different distribution in pollen tubes grown in sucrose-depleted media. Changes were also observed at the cell wall level, particularly in the content and distribution of two enzymes related to cell wall synthesis (sucrose synthase and callose synthase). Furthermore, both callose and newly secreted cell wall material (mainly pectins) showed an altered distribution corresponding to the lack of oscillatory growth in pollen tubes. Growth in glycerol-based media also temporarily affected the movement of generative cells and, in parallel, the deposition of callose plugs.
Conclusion: Pollen tubes represent an ideal model system for studying metabolic pathways during the growth of plant cells. In our study, we found evidence that glycerol, a less energetic source for cell growth than sucrose, causes critical changes in cell wall deposition. The evidence that different aspects of pollen tube growth are affected is an indication that pollen tubes adapt to metabolic stress.
Figures









References
-
- Aloisi I, Cai G, Tumiatti V, Minarini A, Del Duca SD. 2015. Natural polyamines and synthetic analogues modify the growth and the morphology of Pyrus communis pollen tubes affecting ROS levels and causing cell death. Plant Science 239: 92–105. - PubMed
-
- Aubert S, Gout E, Bligny R, Douce R. 1994. Multiple effects of glycerol on plant cell metabolism. Phosphorus-31 nuclear magnetic resonance studies. Journal of Biological Chemistry 269: 21420–21427. - PubMed
-
- Biagini G, Faleri C, Cresti M, Cai G. 2014. Sucrose concentration in the growth medium affects the cell wall composition of tobacco pollen tubes. Plant Reproduction 27: 129–144. - PubMed
-
- Brewbaker JL, Kwack BH. 1963. The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany 50: 859–865.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

