Feasibility study of cancer genome alterations identified by next generation sequencing: ABC study
- PMID: 29659903
- PMCID: PMC5974784
- DOI: 10.1093/jjco/hyy052
Feasibility study of cancer genome alterations identified by next generation sequencing: ABC study
Abstract
Background: To confirm the feasibility and explore the clinical applicability of amplicon sequencing by next generation sequencing (NGS) of biopsy samples from patients with advanced solid tumors, we conducted a prospective study.
Methods: Patients with unresectable, advanced, or recurrent solid tumors were included. Key eligibility criteria were as follows: 20 years or older, any planned systemic therapy, adequate lesion for biopsy, and written informed consent. Samples were fixed in 10% buffered formalin and embedded in paraffin. Cancer-derived DNA was extracted, and amplicon sequencing was performed using Ion AmpliseqTM Cancer Hotspot Panel version 1.0 or version 2.0 by central vendor. We evaluated the success rate of sequencing, and the proportion of the patients with actionable mutations. We organized an expert panel to share the results of targeted sequence, make annotations and reports, and discuss concomitant ethical/legal/social issues.
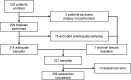
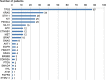
Results: A total of 232 patients were included, and 208 were successfully analyzed (success rate of 89.7%). The biopsy procedures were safe, with only one case of Grade 3 vasovagal reaction. The proportion of actionable/druggable mutations was 38.9% (81/208), which was not significantly different between the cancer panel version 1.0 and version 2.0 (P = 0.476). Expert panel could discuss the findings and make sufficient reports.
Conclusions: We confirmed the feasibility of NGS-based amplicon sequencing using biopsy samples, making the basis for nationwide genome screening for cancer patients using biopsy samples. Our results suggest that focused panel may be sufficient to detect major mutations.
Figures

References
-
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–39. - PubMed
-
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–500. - PubMed
-
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. - PubMed
-
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011–9. - PubMed
-
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697–705. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

