A randomized, double-blind, non-inferiority study of hydromorphone hydrochloride immediate-release tablets versus oxycodone hydrochloride immediate-release powder for cancer pain: efficacy and safety in Japanese cancer patients
- PMID: 29659913
- PMCID: PMC5974780
- DOI: 10.1093/jjco/hyy038
A randomized, double-blind, non-inferiority study of hydromorphone hydrochloride immediate-release tablets versus oxycodone hydrochloride immediate-release powder for cancer pain: efficacy and safety in Japanese cancer patients
Abstract
Background: Hydromorphone is a standard opioid analgesic for cancer pain that, prior to this study, was not approved in Japan, where options for opioid switching are limited. We aimed to investigate the efficacy and safety of hydromorphone (DS-7113b) immediate-release tablets in opioid-naïve cancer patients with moderate to severe cancer pain.
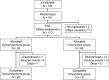
Methods: Multicenter, active-controlled, randomized, double-blind, parallel-group, non-inferiority study of 183 cancer patients over 20 years of age at 50 clinical sites in Japan. Hydromorphone tablets or oxycodone hydrochloride powder was orally administered four times daily for 5 days. The initial doses of hydromorphone and oxycodone hydrochloride were 4 mg/day and 10 mg/day, respectively, and adjusted as necessary. Efficacy was evaluated as the intergroup difference (95% confidence interval [CI]) of the least squares mean by analysis of covariance, using the baseline visual analog scale (VAS) as a covariate for change in VAS score at treatment completion/discontinuation in the full analysis set.
Results: Non-inferiority of hydromorphone versus oxycodone was confirmed, with an intergroup difference (95% CI) in the least squares mean of -3.4 mm (-9.8 to 3.1 mm) for change in VAS scores, which was below the upper limit of the 95% CI at 10 mm, the non-inferiority limit determined during study planning. Adverse events occurred in 83.0% (73/88) of patients in the hydromorphone group and 77.4% (65/84) in the oxycodone group. The most frequently observed adverse events were somnolence, constipation, vomiting and nausea.
Conclusions: The efficacy and safety of hydromorphone tablets are equivalent to those of oxycodone immediate-release powder.
Figures
References
-
- WHO Cancer Pain Relief: With a Guide to Opioid Availability. 2nd edn Geneva: World Health Organization, 1996; apps.who.int/iris/bitstream/10665/37896/1/9241544821.pdf (23 March 2018, date last accessed).
-
- Zech DF, Grond S, Lynch J, et al. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain 1995;63:65–76. - PubMed
-
- Hastie BA, Gilson AM, Maurer MA, Cleary JF. An examination of global and regional opioid consumption trends 1980–2011. J Pain Palliat Care Pharmacother 2014;28:259–75. - PubMed
-
- International Narcotics Control Board Report of the International Narcotics Control Board for 2013. United Nations, New York: International Narcotics Control Board, 2014; www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2013/Part_4... (23 March 2018, date last accessed).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources