Inhibition of OsSWEET11 function in mesophyll cells improves resistance of rice to sheath blight disease
- PMID: 29660235
- PMCID: PMC6638089
- DOI: 10.1111/mpp.12689
Inhibition of OsSWEET11 function in mesophyll cells improves resistance of rice to sheath blight disease
Abstract
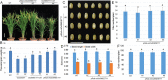
Pathogen-host interaction is a complicated process; pathogens mainly infect host plants to acquire nutrients, especially sugars. Rhizoctonia solani, the causative agent of sheath blight disease, is a major pathogen of rice. However, it is not known how this pathogen obtains sugar from rice plants. In this study, we found that the rice sugar transporter OsSWEET11 is involved in the pathogenesis of sheath blight disease. Quantitative real-time polymerase chain reaction (qRT-PCR) and β-d-glucuronidase expression analyses showed that R. solani infection significantly enhanced OsSWEET11 expression in leaves amongst the clade III SWEET members. The analyses of transgenic plants revealed that Ossweet11 mutants were less susceptible, whereas plants overexpressing OsSWEET11 were more susceptible, to sheath blight compared with wild-type controls, but the yield of OsSWEET11 mutants and overexpressors was reduced. SWEETs become active on oligomerization. Split-ubiquitin yeast two-hybrid, bimolecular fluorescence complementation and co-immunoprecipitation assays showed that mutated OsSWEET11 interacted with normal OsSWEET11. In addition, expression of conserved residue mutated AtSWEET1 inhibited normal AtSWEET1 activity. To analyse whether inhibition of OsSWEET11 function in mesophyll cells is related to defence against this disease, mutated OsSWEET11 was expressed under the control of the Rubisco promoter, which is specific for green tissues. The resistance of transgenic plants to sheath blight disease, but not other disease, was improved, whereas yield production was not obviously affected. Overall, these results suggest that R. solani might acquire sugar from rice leaves by the activation of OsSWEET11 expression. The plants can be protected from infection by manipulation of the expression of OsSWEET11 without affecting the crop yield.
Keywords: OsSWEET11; mesophyll cell; resistance; rice; sheath blight disease.
© 2018 BSPP and John Wiley & Sons Ltd.
Figures





Similar articles
-
Rice oxalate oxidase gene driven by green tissue-specific promoter increases tolerance to sheath blight pathogen (Rhizoctonia solani) in transgenic rice.Mol Plant Pathol. 2013 Dec;14(9):910-22. doi: 10.1111/mpp.12055. Epub 2013 Jul 1. Mol Plant Pathol. 2013. PMID: 23809026 Free PMC article.
-
Tissue-specific expression of Arabidopsis NPR1 gene in rice for sheath blight resistance without compromising phenotypic cost.Plant Sci. 2016 Sep;250:105-114. doi: 10.1016/j.plantsci.2016.06.005. Epub 2016 Jun 5. Plant Sci. 2016. PMID: 27457988
-
Sheath blight resistance in rice is negatively regulated by WRKY53 via SWEET2a activation.Biochem Biophys Res Commun. 2021 Dec 31;585:117-123. doi: 10.1016/j.bbrc.2021.11.042. Epub 2021 Nov 13. Biochem Biophys Res Commun. 2021. PMID: 34801931
-
Understanding sheath blight resistance in rice: the road behind and the road ahead.Plant Biotechnol J. 2020 Apr;18(4):895-915. doi: 10.1111/pbi.13312. Epub 2020 Jan 29. Plant Biotechnol J. 2020. PMID: 31811745 Free PMC article. Review.
-
Advances in breeding, biotechnology, and nanotechnological approaches to combat sheath blight disease in rice.Mol Biol Rep. 2024 Sep 4;51(1):958. doi: 10.1007/s11033-024-09889-5. Mol Biol Rep. 2024. PMID: 39230778 Review.
Cited by
-
Genome-Wide Identification and Expression Profile Analysis of Sugars Will Eventually Be Exported Transporter (SWEET) Genes in Zantedeschia elliottiana and Their Responsiveness to Pectobacterium carotovora subspecies Carotovora (Pcc) Infection.Int J Mol Sci. 2024 Feb 7;25(4):2004. doi: 10.3390/ijms25042004. Int J Mol Sci. 2024. PMID: 38396683 Free PMC article.
-
Suppressing chlorophyll degradation by silencing OsNYC3 improves rice resistance to Rhizoctonia solani, the causal agent of sheath blight.Plant Biotechnol J. 2022 Feb;20(2):335-349. doi: 10.1111/pbi.13715. Epub 2021 Oct 20. Plant Biotechnol J. 2022. PMID: 34582620 Free PMC article.
-
Identification of the SWEET gene family and functional characterization of PsSWEET1a and PsSWEET17b in the regulation of sugar accumulation in 'Fengtang' plum (Prunus salicina Lindl.).BMC Plant Biol. 2025 Apr 1;25(1):407. doi: 10.1186/s12870-025-06407-y. BMC Plant Biol. 2025. PMID: 40165087 Free PMC article.
-
Sugar conundrum in plant-pathogen interactions: roles of invertase and sugar transporters depend on pathosystems.J Exp Bot. 2022 Apr 5;73(7):1910-1925. doi: 10.1093/jxb/erab562. J Exp Bot. 2022. PMID: 35104311 Free PMC article.
-
Ammonium transporter 1 increases rice resistance to sheath blight by promoting nitrogen assimilation and ethylene signalling.Plant Biotechnol J. 2022 Jun;20(6):1085-1097. doi: 10.1111/pbi.13789. Epub 2022 Feb 24. Plant Biotechnol J. 2022. PMID: 35170194 Free PMC article.
References
-
- Chen, H.Y. , Huh, J. , Yu, Y. , Ho, L. , Chen, L. , Tholl, D. , Frommer, W.B. and Guo, W.J. (2015) The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant J. 83, 1046–1058. - PubMed
-
- Chen, L.Q. , Hou, B.H. , Lalonde, S. , Takanaga, H. , Hartung, M.L. , Qu, X.Q. , Guo, W.J. , Kim, J.G. , Underwood, W. , Chaudhuri, B. , Chermak, D. , Antony, G. , White, F.F. , Somerville, S.C. , Mudgett, M.B. and Frommer, W.B. (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature, 468, 527–532. - PMC - PubMed
-
- Chen, L.Q. , Qu, X.Q. , Hou, B.H. , Sosso, D. , Osorio, S. , Fernie, A.R. and Frommer, W.B. (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science, 335, 207–211. - PubMed
-
- Chen, L.Q. , Cheung, L.S. , Feng, L. , Tanner, W. and Frommer, W.B. (2015) Transport of sugars. Annu. Rev. Biochem. 84, 865–894. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

