Oxylipins from both pathogen and host antagonize jasmonic acid-mediated defence via the 9-lipoxygenase pathway in Fusarium verticillioides infection of maize
- PMID: 29660236
- PMCID: PMC6638020
- DOI: 10.1111/mpp.12690
Oxylipins from both pathogen and host antagonize jasmonic acid-mediated defence via the 9-lipoxygenase pathway in Fusarium verticillioides infection of maize
Abstract
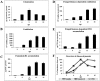
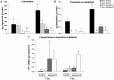
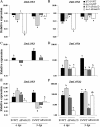
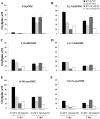
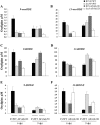
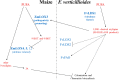
Oxylipins are a newly emerging group of signals that serve defence roles or promote virulence. To identify specific host and fungal genes and oxylipins governing the interactions between maize and Fusarium verticillioides, maize wild-type and lipoxygenase3 (lox3) mutant were inoculated with either F. verticillioides wild-type or linoleate-diol-synthase 1-deleted mutant (ΔFvlds1D). The results showed that lox3 mutants were more resistant to F. verticillioides. The reduced colonization on lox3 was associated with reduced fumonisin production and with a stronger and earlier induction of ZmLOX4, ZmLOX5 and ZmLOX12. In addition to the reported defence function of ZmLOX12, we showed that lox4 and lox5 mutants were more susceptible to F. verticillioides and possessed decreased jasmonate levels during infection, suggesting that these genes are essential for jasmonic acid (JA)-mediated defence. Oxylipin profiling revealed a dramatic reduction in fungal linoleate diol synthase 1 (LDS1)-derived oxylipins, especially 8-HpODE (8-hydroperoxyoctadecenoic acid), in infected lox3 kernels, indicating the importance of this molecule in virulence. Collectively, we make the following conclusions: (1) LOX3 is a major susceptibility factor induced by fungal LDS1-derived oxylipins to suppress JA-stimulating 9-LOXs; (2) LOX3-mediated signalling promotes the biosynthesis of virulence-promoting oxylipins in the fungus; and (3) both fungal LDS1- and host LOX3-produced oxylipins are essential for the normal infection and colonization processes of maize seed by F. verticillioides.
Keywords: Fusarium; jasmonic acid; linoleate diol synthase; maize; oxylipin cross-talk; susceptibility genes.
© 2018 BSPP and John Wiley & Sons Ltd.
Figures


References
-
- Andreou, A. , Brodhun, F. and Feussner, I. (2009) Biosynthesis of oxylipins in non‐mammals. Prog. Lipid Res. 48, 148–170. - PubMed
-
- Bacon, C.W. , Hinton, D.M. , Porter, J.K. , Glenn, A.E. and Kuldau, G. (2004) Fusaric acid, a Fusarium verticillioides metabolite, antagonistic to the endophytic biocontrol bacterium Bacillus mojavensis . Can. J. Bot. 82, 878–885.
-
- Battilani, P. , Rossi, V. and Pietri, A. (2003) Modelling Fusarium verticillioides infection and fumonisin synthesis in maize ears. Asp. Appl. Biol. 68, 91–100.
-
- Battilani, P. , Pietri, A. , Barbano, C. , Scandolara, A. , Bertuzzi, T. and Marocco, A. (2008) Logistic regression modeling of cropping systems to predict fumonisin contamination in maize. J. Agric. Food Chem. 56, 10 433–10 438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

