IDH2 deficiency accelerates skin pigmentation in mice via enhancing melanogenesis
- PMID: 29660504
- PMCID: PMC6006679
- DOI: 10.1016/j.redox.2018.04.008
IDH2 deficiency accelerates skin pigmentation in mice via enhancing melanogenesis
Abstract
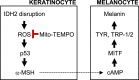
Melanogenesis is a complex biosynthetic pathway regulated by multiple agents, which are involved in the production, transport, and release of melanin. Melanin has diverse roles, including determination of visible skin color and photoprotection. Studies indicate that melanin synthesis is tightly linked to the interaction between melanocytes and keratinocytes. α-melanocyte-stimulating hormone (α-MSH) is known as a trigger that enhances melanin biosynthesis in melanocytes through paracrine effects. Accumulated reactive oxygen species (ROS) in skin affects both keratinocytes and melanocytes by causing DNA damage, which eventually leads to the stimulation of α-MSH production. Mitochondria are one of the main sources of ROS in the skin and play a central role in modulating redox-dependent cellular processes such as metabolism and apoptosis. Therefore, mitochondrial dysfunction may serve as a key for the pathogenesis of skin melanogenesis. Mitochondrial NADP+-dependent isocitrate dehydrogenase (IDH2) is a key enzyme that regulates mitochondrial redox balance and reduces oxidative stress-induced cell injury through the generation of NADPH. Downregulation of IDH2 expression resulted in an increase in oxidative DNA damage in mice skin through ROS-dependent ATM-mediated p53 signaling. IDH2 deficiency also promoted pigmentation on the dorsal skin of mice, as evident from the elevated levels of melanin synthesis markers. Furthermore, pretreatment with mitochondria-targeted antioxidant mito-TEMPO alleviated oxidative DNA damage and melanogenesis induced by IDH2 deficiency both in vitro and in vivo. Together, our findings highlight the role of IDH2 in skin melanogenesis in association with mitochondrial ROS and suggest unique therapeutic strategies for the prevention of skin pigmentation.
Keywords: IDH2; Melanogenesis; Mito-TEMPO; Mitochondria; α-MSH.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




Similar articles
-
IDH2 deficiency impairs cutaneous wound healing via ROS-dependent apoptosis.Biochim Biophys Acta Mol Basis Dis. 2019 Nov 1;1865(11):165523. doi: 10.1016/j.bbadis.2019.07.017. Epub 2019 Jul 31. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31376482
-
Mitochondrial NADP+-dependent isocitrate dehydrogenase deficiency increases cisplatin-induced oxidative damage in the kidney tubule cells.Cell Death Dis. 2018 May 1;9(5):488. doi: 10.1038/s41419-018-0537-6. Cell Death Dis. 2018. PMID: 29695796 Free PMC article.
-
IDH2 deficiency exacerbates acetaminophen hepatotoxicity in mice via mitochondrial dysfunction-induced apoptosis.Biochim Biophys Acta Mol Basis Dis. 2019 Sep 1;1865(9):2333-2341. doi: 10.1016/j.bbadis.2019.05.012. Epub 2019 May 20. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31121248
-
Does alpha-MSH have a role in regulating skin pigmentation in humans?Pigment Cell Res. 1998 Oct;11(5):265-74. doi: 10.1111/j.1600-0749.1998.tb00735.x. Pigment Cell Res. 1998. PMID: 9877097 Review.
-
MC1R: Front and Center in the Bright Side of Dark Eumelanin and DNA Repair.Int J Mol Sci. 2018 Sep 8;19(9):2667. doi: 10.3390/ijms19092667. Int J Mol Sci. 2018. PMID: 30205559 Free PMC article. Review.
Cited by
-
IDH2: A novel biomarker for environmental exposure in blood circulatory system disorders.Oncol Lett. 2022 Jun 24;24(2):278. doi: 10.3892/ol.2022.13398. eCollection 2022 Aug. Oncol Lett. 2022. PMID: 35814829 Free PMC article. Review.
-
Human Tyrosinase Displayed on the Surface of Chinese Hamster Ovary Cells for Ligand Fishing of Tyrosinase Inhibitors from Medicinal Plants.Molecules. 2024 Dec 25;30(1):30. doi: 10.3390/molecules30010030. Molecules. 2024. PMID: 39795088 Free PMC article.
-
Topical application of Tea leaf-derived nanovesicles reduce melanogenesis by modulating the miR-828b/MYB4 axis: better permeability and therapeutic efficacy than conventional tea extracts.Mater Today Bio. 2025 Jul 17;34:102108. doi: 10.1016/j.mtbio.2025.102108. eCollection 2025 Oct. Mater Today Bio. 2025. PMID: 40735705 Free PMC article.
-
Skin transcriptomic and selection signature analyses identify ASIP as a key gene in cattle coat color determination.Front Genet. 2025 Apr 28;16:1577647. doi: 10.3389/fgene.2025.1577647. eCollection 2025. Front Genet. 2025. PMID: 40357365 Free PMC article.
-
Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction.Plants (Basel). 2022 Dec 22;12(1):65. doi: 10.3390/plants12010065. Plants (Basel). 2022. PMID: 36616194 Free PMC article.
References
-
- Plonka P.M., Passeron T., Brenner M., Tobin D.J., Shibahara S., Thomas A., Slominski A., Kadekaro A.L., Hershkovitz D., Peters E., Nordlund J.J., Abdel-Malek Z., Takeda K., Paus R., Ortonne J.P., Hearing V.J., Schallreuter K.U. What are melanocytes really doing all day long…? Exp. Dermatol. 2009;18:799–819. - PMC - PubMed
-
- Herrling T., Jung K., Fuchs J. The role of melanin as protector against free radicals in skin and ints role as free radical indicator in hair. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008;69:1429–1435. - PubMed
-
- Delevoye C. Melanin transfer: the keratinocytes are more than gluttons. J. Invest. Dermatol. 2014;134:877–879. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

