Caffeine-catalyzed gels
- PMID: 29660635
- PMCID: PMC5937912
- DOI: 10.1016/j.biomaterials.2018.04.010
Caffeine-catalyzed gels
Abstract
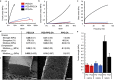
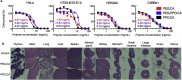
Covalently cross-linked gels are utilized in a broad range of biomedical applications though their synthesis often compromises easy implementation. Cross-linking reactions commonly utilize catalysts or conditions that can damage biologics and sensitive compounds, producing materials that require extensive post processing to achieve acceptable biocompatibility. As an alternative, we report a batch synthesis platform to produce covalently cross-linked materials appropriate for direct biomedical application enabled by green chemistry and commonly available food grade ingredients. Using caffeine, a mild base, to catalyze anhydrous carboxylate ring-opening of diglycidyl-ether functionalized monomers with citric acid as a tri-functional crosslinking agent we introduce a novel poly(ester-ether) gel synthesis platform. We demonstrate that biocompatible Caffeine Catalyzed Gels (CCGs) exhibit dynamic physical, chemical, and mechanical properties, which can be tailored in shape, surface texture, solvent response, cargo release, shear and tensile strength, among other potential attributes. The demonstrated versatility, low cost and facile synthesis of these CCGs renders them appropriate for a broad range of customized engineering applications including drug delivery constructs, tissue engineering scaffolds, and medical devices.
Keywords: Biocompatible materials; Green-chemistry; Shape-changing thermosets.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Hennink W.E., van Nostrum C.F. Adv. Drug Deliv. Rev. 2002;54:13. - PubMed
-
- Caló E., Khutoryanskiy V.V. Biomedical applications of hydrogels: a review of patents and commercial products. Eur. Polym. J. 2015;65:252–267.
-
- Mastropietro D.J., Omidian H., Park K. Drug delivery applications for superporous hydrogels. Expet Opin. Drug Deliv. 2012;9:71–89. - PubMed
-
- Pereira M.J.N., Ouyang B., Sundback C.A., Lang N., Friehs I., Mureli S., Pomerantseva I., McFadden J., Mochel M.C., Mwizerwa O., del Nido P., Sarkar D., Masiakos P.T., Langer R., Ferreira L.S., Karp J.M. A highly tunable biocompatible and multifunctional biodegradable elastomer. Adv. Mater. 2012;25:1209–1215. - PMC - PubMed
-
- Behl M., Razzaq M.Y., Lendlein A. Thermal responsive shape memory polymers for biomedical applications. Adv. Mater. 2010;22:3388–3410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

