Final results of a phase 2, open-label study of indisulam, idarubicin, and cytarabine in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome
- PMID: 29660836
- PMCID: PMC6800041
- DOI: 10.1002/cncr.31398
Final results of a phase 2, open-label study of indisulam, idarubicin, and cytarabine in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome
Abstract
Background: Indisulam possesses anticancer properties through down-regulation of various cell-cycle checkpoint molecules, thereby blocking the phosphorylation of retinoblastoma protein and inducing p53 and p21. Indisulam exhibits synergy with nucleoside analogs and topoisomerase inhibitors.
Methods: The authors designed a phase 2 study of indisulam in combination with idarubicin and cytarabine in patients who had relapsed/refractory acute myeloid leukemia AML and high-risk myelodysplastic syndrome. In stage 1, patients received intravenous indisulam at 400 mg/m2 on days 1 and 8 of a 28-day cycle. If they had no response, then patients received same dose schedule of indisulam followed by intravenous idarubicin 8 mg/m2 daily for 3 days and cytarabine 1.0 g/m2 over 24 hours daily on days 9 through 12 (for those aged < 60 years) or days 9 through 11 (for those aged > 60 years) of a 28-day cycle. Primary endpoints included the overall response rate, and secondary objectives included overall survival.
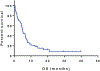
Results: Forty patients were enrolled. Of the 37 evaluable patients, 31 received indisulam with chemotherapy. Of these, 11 (35%) responded for a median duration of 5.3 months. The estimated 1-year overall survival rate was 51% for responders compared with 8 % for nonresponders (P < .001). The most common grade ≥3 nonhematologic toxicities were electrolyte abnormalities (50%) and febrile neutropenia (28%).
Conclusions: The combination of indisulam with idarubicin and cytarabine yielded a 35% response rate in heavily pretreated patients with AML. With emerging data identifying the expression of DCAF15 (DDB1 and CUL4-associated factor 15) as a potential biomarker for activity, the combination of indisulam with idarubicin and cytarabine should be studied in a biomarker-driven trial or in patients who have splicing factor mutations. Cancer 2018;124:2758-65. © 2018 American Cancer Society. Cancer 2018;124:2758-2765. © 2018 American Cancer Society.
Keywords: acute myeloid leukemia (AML); biomarker; cytarabine; idarubicin; indisulam; relapsed/refractory.
© 2018 American Cancer Society.
Conflict of interest statement
Conflicts of Interest: None
Figures
References
-
- Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23: 1969–1978. - PubMed
-
- Owa T, Yoshino H, Okauchi T, et al. Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J Med Chem. 1999;42: 3789–3799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous