Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity
- PMID: 29661791
- PMCID: PMC6396335
- DOI: 10.1101/cshperspect.a028480
Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity
Abstract
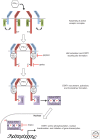
Originally identified in studies of cellular resistance to viral infection, interferon (IFN)-γ is now known to represent a distinct member of the IFN family and plays critical roles not only in orchestrating both innate and adaptive immune responses against viruses, bacteria, and tumors, but also in promoting pathologic inflammatory processes. IFN-γ production is largely restricted to T lymphocytes and natural killer (NK) cells and can ultimately lead to the generation of a polarized immune response composed of T helper (Th)1 CD4+ T cells and CD8+ cytolytic T cells. In contrast, the temporally distinct elaboration of IFN-γ in progressively growing tumors also promotes a state of adaptive resistance caused by the up-regulation of inhibitory molecules, such as programmed-death ligand 1 (PD-L1) on tumor cell targets, and additional host cells within the tumor microenvironment. This review focuses on the diverse positive and negative roles of IFN-γ in immune cell activation and differentiation leading to protective immune responses, as well as the paradoxical effects of IFN-γ within the tumor microenvironment that determine the ultimate fate of that tumor in a cancer-bearing individual.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Abed NS, Chace JH, Fleming AL, Cowdery JS. 1994. Interferon-γ regulation of B lymphocyte differentiation: Activation of B cells is a prerequisite for IFN-γ-mediated inhibition of B cell differentiation. Cell Immunol 153: 356–366. - PubMed
-
- Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. 2002. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol 3: 549–557. - PubMed
-
- Agnello D, Lankford CSR, Bream J, Morinobu A, Gadina M, O’Shea JJ, Frucht DM. 2003. Cytokines and transcription factors that regulate T helper cell differentiation: New players and new insights. J Clin Immunol 23: 147–161. - PubMed
-
- Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, et al. 1999. SOCS1 is a critical inhibitor of interferon γ signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98: 597–608. - PubMed
-
- Arakawa T, Hsu YR, Parker CG, Lai PH. 1986. Role of polycationic C-terminal portion in the structure and activity of recombinant human interferon-γ. J Biol Chem 261: 8534–8539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
