Triclosan Is an Aminoglycoside Adjuvant for Eradication of Pseudomonas aeruginosa Biofilms
- PMID: 29661867
- PMCID: PMC5971565
- DOI: 10.1128/AAC.00146-18
Triclosan Is an Aminoglycoside Adjuvant for Eradication of Pseudomonas aeruginosa Biofilms
Abstract
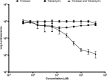
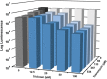
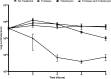
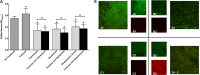
One of the most important clinical obstacles in cystic fibrosis (CF) treatment is antibiotic treatment failure due to biofilms produced by Pseudomonas aeruginosa The ability of this pathogen to survive eradication by tobramycin and pathoadapt into a hyperbiofilm state leading to chronic infections is key to its success. Retrospective studies have demonstrated that preventing this pathoadaptation by improving eradication is essential to extend the lives of CF patients. To identify adjuvants that enhance tobramycin eradication of P. aeruginosa, we performed a high-throughput screen of 6,080 compounds from four drug-repurposing libraries. We identified that the Food and Drug Administration (FDA)-approved compound triclosan, in combination with tobramycin, resulted in a 100-fold reduction of viable cells within biofilms at 6 h, but neither compound alone had significant antimicrobial activity against biofilms. This synergistic treatment significantly accelerated the killing of biofilms compared to that with tobramycin treatment alone, and the combination was effective against 6/7 CF clinical isolates compared to tobramycin treatment alone, including a tobramycin-resistant strain. Further, triclosan and tobramycin killed persister cells, causing a 100-fold reduction by 8 h and complete eradication by 24 h. Triclosan also enhances tobramycin killing of multiple Burkholderia cenocepacia and Staphylococcus aureus clinical isolates grown as biofilms. Additionally, triclosan showed synergy with other aminoglycosides, such as gentamicin or streptomycin. Triclosan is a well-tolerated aminoglycoside adjuvant shown to be safe for human use that could improve the treatment of biofilm-based infections.
Keywords: Pseudomonas aeruginosa; biofilm; persister; tobramycin; triclosan.
Copyright © 2018 American Society for Microbiology.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

