Characterizing Blood-Stage Antimalarial Drug MIC Values In Vivo Using Reinfection Patterns
- PMID: 29661873
- PMCID: PMC6021672
- DOI: 10.1128/AAC.02476-17
Characterizing Blood-Stage Antimalarial Drug MIC Values In Vivo Using Reinfection Patterns
Abstract
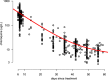
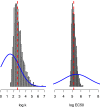
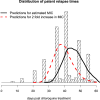
The MIC is an essential quantitative measure of the asexual blood-stage effect of an antimalarial drug. In areas of high malaria transmission, and thus frequent individual infection, patients who are treated with slowly eliminated antimalarials become reinfected as drug concentrations decline. In the frequent relapse forms of Plasmodium vivax and in Plasmodium ovale malaria, recurrent infection occurs from relapses which begin to emerge from the liver approximately 2 weeks after the primary illness. An important determinant of the interval from starting treatment of a symptomatic infection to the patency of these recurrent infections is the in vivo concentration-response relationship and thus the in vivo MIC. Using mechanistic knowledge of parasite asexual replication and the pharmacokinetic and pharmacodynamic properties of the antimalarial drugs, a generative statistical model was derived which relates the concentration-response relationship to time of reinfection patency. This model was used to estimate the in vivo MIC of chloroquine in the treatment of Plasmodium vivax malaria.
Keywords: MIC; antimalarial agents; chloroquine; pharmacodynamics; pharmacokinetics.
Copyright © 2018 Watson et al.
Figures


References
-
- Beadle C, McElroy PD, Oster CN, Beier JC, Oloo AJ, Onyango FK, Chumo DK, Bales JD, Sherwood JA, Hoffman SL. 1995. Impact of transmission intensity and age on Plasmodium falciparum density and associated fever: implications for malaria vaccine trial design. J Infect Dis 172:1047–1054. doi:10.1093/infdis/172.4.1047. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

