Interleukin-22 Immunotherapy during Severe Influenza Enhances Lung Tissue Integrity and Reduces Secondary Bacterial Systemic Invasion
- PMID: 29661933
- PMCID: PMC6013680
- DOI: 10.1128/IAI.00706-17
Interleukin-22 Immunotherapy during Severe Influenza Enhances Lung Tissue Integrity and Reduces Secondary Bacterial Systemic Invasion
Abstract
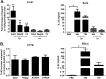
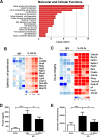
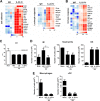
Severe bacterial (pneumococcal) infections are commonly associated with influenza and are significant contributors to the excess morbidity and mortality of influenza. Disruption of lung tissue integrity during influenza participates in bacterial pulmonary colonization and dissemination out of the lungs. Interleukin-22 (IL-22) has gained considerable interest in anti-inflammatory and anti-infection immunotherapy over the last decade. In the current study, we investigated the effect of exogenous IL-22 delivery on the outcome of pneumococcal superinfection postinfluenza. Our data show that exogenous treatment of influenza virus-infected mice with recombinant IL-22 reduces bacterial dissemination out of the lungs but is without effect on pulmonary bacterial burden. Reduced systemic bacterial dissemination was linked to reinforced pulmonary barrier functions, as revealed by total protein measurement in the bronchoalveolar fluids, intratracheal fluorescein isothiocyanate-dextran tracking, and histological approaches. We describe an IL-22-specific gene signature in the lung tissue of influenza A virus (IAV)-infected (and naive) mice that might explain the observed effects. Indeed, exogenous IL-22 modulates the gene expression profile in a way that suggests reinforcement of tissue integrity. Our results open the way to alternative approaches for limiting postinfluenza bacterial superinfection, particularly, systemic bacterial invasion.
Keywords: epithelial barrier; influenza; interleukin 22; superinfection.
Copyright © 2018 American Society for Microbiology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

