Dual functionality of β-tryptase protomers as both proteases and cofactors in the active tetramer
- PMID: 29661938
- PMCID: PMC6016454
- DOI: 10.1074/jbc.M117.812016
Dual functionality of β-tryptase protomers as both proteases and cofactors in the active tetramer
Abstract
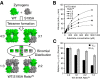
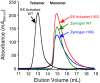
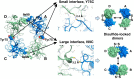
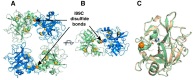
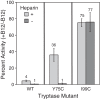
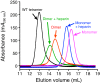
Human β-tryptase, a tetrameric trypsin-like serine protease, is an important mediator of the allergic inflammatory responses in asthma. During acute hypersensitivity reactions, mast cells degranulate, releasing active tetramer as a complex with proteoglycans. Extensive efforts have focused on developing therapeutic β-tryptase inhibitors, but its unique activation mechanism is less well-explored. Tryptase is active only after proteolytic removal of the pro-domain followed by tetramer formation via two distinct symmetry-related interfaces. We show that the cleaved I16G mutant cannot tetramerize, likely due to impaired insertion of its N terminus into its "activation pocket," indicating allosteric linkage at multiple sites on each protomer. We engineered cysteines into each of the two distinct interfaces (Y75C for small or I99C for large) to assess the activity of each tetramer and disulfide-locked dimer. Using size-exclusion chromatography and enzymatic assays, we demonstrate that the two large tetramer interfaces regulate enzymatic activity, elucidating the importance of this protein-protein interaction for allosteric regulation. Notably, the I99C large interface dimer is active, even in the absence of heparin. We show that a monomeric β-tryptase mutant (I99C*/Y75A/Y37bA, where C* is cysteinylated Cys-99) cannot form a dimer or tetramer, yet it is active but only in the presence of heparin. Thus heparin both stabilizes the tetramer and allosterically conditions the active site. We hypothesize that each β-tryptase protomer in the tetramer has two distinct roles, acting both as a protease and as a cofactor for its neighboring protomer, to allosterically regulate enzymatic activity, providing a rationale for direct correlation of tetramer stability with proteolytic activity.
Keywords: allosteric regulation; enzyme mechanism; heparin-binding protein; protein engineering; protein–protein interaction; serine protease; tetramerization; tryptase.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors were all employees of Genentech, Inc., when the work was carried out except for L. B. S. L. B. S. is a paid consultant for Genentech, Inc
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

