A Firefly Luciferase Dual Color Bioluminescence Reporter Assay Using Two Substrates To Simultaneously Monitor Two Gene Expression Events
- PMID: 29662072
- PMCID: PMC5902630
- DOI: 10.1038/s41598-018-24278-2
A Firefly Luciferase Dual Color Bioluminescence Reporter Assay Using Two Substrates To Simultaneously Monitor Two Gene Expression Events
Abstract
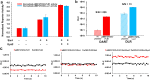
Effective methods for monitoring eukaryotic gene expression and regulation based on bioluminescence - the emission of light by living organisms - are well established. Typically, the expression of a gene of interest is reported on with high sensitivity and over a wide dynamic range by the emission of light from a variety of engineered luciferase genes from beetles and marine organisms. The luciferase reporter genes are expressed downstream of the target gene or promoter and detected after exogenous addition of luciferin substrates. We describe a novel bioluminescence reporter method for the simultaneous monitoring of two genes expressing engineered firefly luciferase variants that emit readily distinguishable green and red light signals. The key feature is the selectivity of the enzymes for two luciferin substrates that determine each emission color. To validate our method, we performed a complex promoter transactivation experiment side-by-side with the Dual-Luciferase Reporter protocol and obtained essentially identical results. Additional comparative experiments demonstrated that our assay system provided improvements in background, cell normalization, and detectability compared to representative available methods. With access to a luminometer equipped with two optical filters, this method is an excellent choice for genetic reporter assays that can be performed with a single reagent solution.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Cevenini, L., Calabretta, M. M., Calabria, D., Roda, A. & Michelini, E. In Bioluminescence: Fundamentals and Applications in Biotechnology, Vol 3, Vol. 154. (eds G. Thouand & R. Marks) 3–17 (2016).
-
- Schagat, T., Gaguio, A. & Kopish, K. Normalizing genetic reporter assays: approaches and considerations for increasing consistency and statistical significance. Cell Notes, 9–12 (2007).
-
- Dual-Luciferase® Reporter Assay System Technical Manual No. TM040 (Promega Corporation, 2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

