Long Non-Coding RNA HOXA Transcript Antisense RNA Myeloid-Specific 1-HOXA1 Axis Downregulates the Immunosuppressive Activity of Myeloid-Derived Suppressor Cells in Lung Cancer
- PMID: 29662483
- PMCID: PMC5890118
- DOI: 10.3389/fimmu.2018.00473
Long Non-Coding RNA HOXA Transcript Antisense RNA Myeloid-Specific 1-HOXA1 Axis Downregulates the Immunosuppressive Activity of Myeloid-Derived Suppressor Cells in Lung Cancer
Erratum in
-
Corrigendum: Long Non-Coding RNA HOXA Transcript Antisense RNA Myeloid-Specific 1-HOXA1 Axis Downregulates the Immunosuppressive Activity of Myeloid-Derived Suppressor Cells in Lung Cancer.Front Immunol. 2020 Jan 21;10:2929. doi: 10.3389/fimmu.2019.02929. eCollection 2019. Front Immunol. 2020. PMID: 32038606 Free PMC article.
Abstract
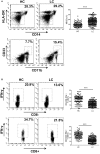
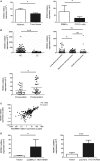
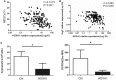
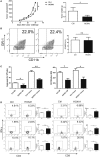
HOXA transcript antisense RNA myeloid-specific 1 (HOTAIRM1) is a long non-coding RNA that has been shown to be a key regulator of myeloid cell development by targeting HOXA1. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells that possess immunosuppressive function. However, the impact of HOTAIRM1 on the development of MDSCs remains unknown. In this study, we demonstrated that HOTAIRM1 was expressed in MDSCs and that overexpression of HOTAIRM1 could downregulate the expression of suppressive molecules in MDSCs. In addition, HOTAIRM1 levels were observed to be decreased in the peripheral blood cells of lung cancer patients compared with those of healthy controls. By analyzing HOTAIRM1 expression levels in different types of lung cancer, we found that HOTAIRM1 was mainly expressed in lung adenocarcinoma. Finally, it was confirmed that HOTAIRM1 could enhance the expression of HOXA1 in MDSCs and that high levels of HOXA1, the target gene of HOTAIRM1, could delay tumor progression and enhance the antitumor immune response by downregulating the immunosuppression of MDSCs. Taken together, this study illustrates that HOTAIRM1/HOXA1 downregulates the immunosuppressive function of MDSCs and may be a potential therapeutic target in lung cancer.
Keywords: HOXA transcript antisense RNA myeloid-specific 1; HOXA1; long non-coding RNA; lung cancer; myeloid-derived suppressor cells.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

