Our Environment Shapes Us: The Importance of Environment and Sex Differences in Regulation of Autoantibody Production
- PMID: 29662485
- PMCID: PMC5890161
- DOI: 10.3389/fimmu.2018.00478
Our Environment Shapes Us: The Importance of Environment and Sex Differences in Regulation of Autoantibody Production
Abstract
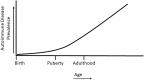
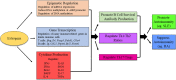
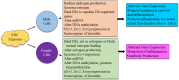
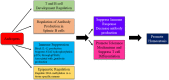
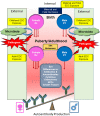
Consequential differences exist between the male and female immune systems' ability to respond to pathogens, environmental insults or self-antigens, and subsequent effects on immunoregulation. In general, females when compared with their male counterparts, respond to pathogenic stimuli and vaccines more robustly, with heightened production of antibodies, pro-inflammatory cytokines, and chemokines. While the precise reasons for sex differences in immune response to different stimuli are not yet well understood, females are more resistant to infectious diseases and much more likely to develop autoimmune diseases. Intrinsic (i.e., sex hormones, sex chromosomes, etc.) and extrinsic (microbiome composition, external triggers, and immune modulators) factors appear to impact the overall outcome of immune responses between sexes. Evidence suggests that interactions between environmental contaminants [e.g., endocrine disrupting chemicals (EDCs)] and host leukocytes affect the ability of the immune system to mount a response to exogenous and endogenous insults, and/or return to normal activity following clearance of the threat. Inherently, males and females have differential immune response to external triggers. In this review, we describe how environmental chemicals, including EDCs, may have sex differential influence on the outcome of immune responses through alterations in epigenetic status (such as modulation of microRNA expression, gene methylation, or histone modification status), direct and indirect activation of the estrogen receptors to drive hormonal effects, and differential modulation of microbial sensing and composition of host microbiota. Taken together, an intriguing question develops as to how an individual's environment directly and indirectly contributes to an altered immune response, dysregulation of autoantibody production, and influence autoimmune disease development. Few studies exist utilizing well-controlled cohorts of both sexes to explore the sex differences in response to EDC exposure and the effects on autoimmune disease development. Translational studies incorporating multiple environmental factors in animal models of autoimmune disease are necessary to determine the interrelationships that occur between potential etiopathological factors. The presence or absence of autoantibodies is not a reliable predictor of disease. Therefore, future studies should incorporate all the susceptibility/influencing factors, coupled with individual genomics, epigenomics, and proteomics, to develop a model that better predicts, diagnoses, and treats autoimmune diseases in a personalized-medicine fashion.
Keywords: DNA methylation; endocrine disrupting chemicals; epigenetics; lupus; microbiota; sex hormones.
Figures

References
-
- Karpuzoglu E, Fenaux JB, Phillips RA, Lengi AJ, Elvinger F, Ansar Ahmed S. Estrogen up-regulates inducible nitric oxide synthase, nitric oxide, and cyclooxygenase-2 in splenocytes activated with T cell stimulants: role of interferon-gamma. Endocrinology (2006) 147(2):662–71. 10.1210/en.2005-0829 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

