A comparison of combined oral contraceptives containing chlormadinone acetate versus drospirenone for the treatment of acne and dysmenorrhea: a randomized trial
- PMID: 29662684
- PMCID: PMC5891982
- DOI: 10.1186/s40834-018-0058-9
A comparison of combined oral contraceptives containing chlormadinone acetate versus drospirenone for the treatment of acne and dysmenorrhea: a randomized trial
Abstract
Background: Oral contraceptives (OCs), aside from contraceptive efficacy, have been widely known for their non-contraceptive benefits. Different progestogens component of the OCs have been shown to improve the skin, hair, menstrual cycle related disorders and dysmenorrhoeic pain. Thus, we compared the efficacy of OCs containing ethinyl estradiol (EE) and chlormadinone acetate (CMA) versus OCs containing EE and drospirenone (DRSP) for the treatment of acne and dysmenorrhea.
Methods: This study was an investigator-blinded, randomized, parallel group study conducted at the Family Planning Clinic, Department of Obstetrics and Gynaecology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Women aged between 18 and 45 years were randomly assigned into two treatment groups, either EE/CMA at the dosage of 30 mcg/2 mg once daily (OD) or EE/DRSP at the dosage of 30 mcg/3 mg OD. The subjects were evaluated for the OC's efficacy for the treatment of acne and dysmenorrhea at baseline visit and after 1, 3, and 6 months of treatment.
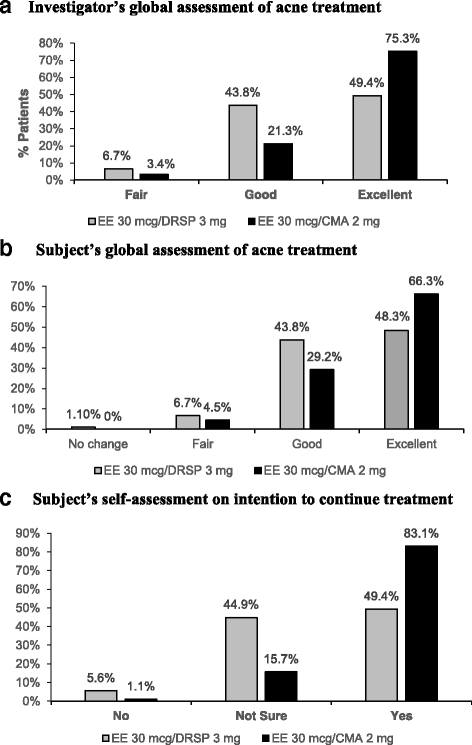
Results: A total of 180 women were randomized into the study. Each group had 90 women. Baseline characteristics between both groups were comparable. At Month 6, there was a significantly greater reduction of total acne lesion in the EE/CMA group than EE/DRSP (72.2% vs 64.5%; p = 0.009). As per the investigator's global assessment of acne treatment, a higher proportion of the subjects from the EE/CMA group was rated "excellent" than those from the EE/DRSP (75.3% vs 49.4%). More subjects from the EE/CMA group had graded their improvement in acne as "excellent" compared to the EE/DRSP group (66.3% vs 48.3%). A higher proportion of the subjects in the EE/CMA group reported a decrease in dysmenorrhoeic pain as "much decrease" and "decrease". The absence of dysmenorrhea pain was more frequently found in the EE/CMA group and significantly seen as early as Month 1 also in the EE/CMA group compared to EE/DRSP (47.2% vs 27.3%, respectively). The treatments were generally well-tolerated in both groups. There were no significant differences between both groups for adverse events.
Conclusions: EE/CMA is more effective for the treatment of acne and dysmenorrhea in women with mild to moderate acne vulgaris and dysmenorrhea than EE/DRSP.
Trial registration: Thai Clinical Trial Registry ID: TCTR20170518001 (date of registration: May 17, 2017; retrospectively registered).
Keywords: Acne; Chlormadinone acetate; Drospirenone; Dysmenorrhea; Oral contraceptive.
Conflict of interest statement
The study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB No. 498/55) and was conducted in accordance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Signed informed consent were obtained from every patient prior to study enrollment.Not applicableThe authors declare that they have no competing interests.
Figures

References
-
- Rabe T, Runnebaum B. The future of oral hormonal contraception: fertility control — update and trends. 1999.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

