Large Scale In Vivo Recording of Sensory Neuron Activity with GCaMP6
- PMID: 29662940
- PMCID: PMC5898788
- DOI: 10.1523/ENEURO.0417-17.2018
Large Scale In Vivo Recording of Sensory Neuron Activity with GCaMP6
Abstract
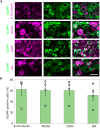
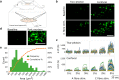
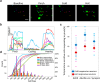
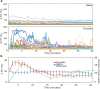
Greater emphasis on the study of intact cellular networks in their physiological environment has led to rapid advances in intravital imaging of the central nervous system (CNS), while the peripheral system remains largely unexplored. To assess large networks of sensory neurons, we selectively label primary afferents with GCaMP6s in male and female C57bl/6 mice and visualize their functional responses to peripheral stimulation in vivo. We show that we are able to monitor the activity of hundreds of sensory neurons simultaneously, with sufficient sensitivity to detect, in most cases, single action potentials with a typical rise time of around 200 ms, and an exponential decay with a time constant of approximately 700 ms. With this technique we are able to characterize the responses of large populations of sensory neurons to innocuous and noxious mechanical and thermal stimuli under normal and inflammatory conditions. We demonstrate that the majority of primary afferents are polymodal with between 50-80% of thermally sensitive DRG neurons responding also to noxious mechanical stimulation. We also specifically assess the small population of peripheral cold neurons and demonstrate significant sensitization to cooling after a model of sterile and persistent inflammation, with significantly increased sensitivity already at decreases of 5°C when compared to uninflamed responses. This not only reveals interesting new insights into the (patho)physiology of the peripheral nervous system but also demonstrates the sensitivity of this imaging technique to physiological changes in primary afferents.
Keywords: dorsal root ganglia; genetically encoded calcium indicators; in vivo imaging; nociception; pain; primary afferents.
Figures


References
-
- Bewersdorf J, Pick R, Hell SW (1998) Multifocal multiphoton microscopy. Opt Lett 23:655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
