Prediction and diagnosis of interval metastasis after neoadjuvant chemoradiotherapy for oesophageal cancer using 18F-FDG PET/CT
- PMID: 29663014
- PMCID: PMC6097755
- DOI: 10.1007/s00259-018-4011-6
Prediction and diagnosis of interval metastasis after neoadjuvant chemoradiotherapy for oesophageal cancer using 18F-FDG PET/CT
Abstract
Objective: During neoadjuvant chemoradiotherapy for oesophageal cancer, or in the interval prior to surgery, some patients develop systemic metastasis. This study aimed to evaluate the diagnostic performance of 18F-FDG PET/CT for the detection of interval metastasis and to identify predictors of interval metastases in a large cohort of oesophageal cancer patients.
Methods: In total, 783 consecutive patients with potentially resectable oesophageal cancer who underwent chemoradiotherapy and pre- and post-treatment 18F-FDG PET/CT between 2006 and 2015 were analyzed from a prospectively maintained database. Diagnostic accuracy measures were calculated on a per-patient basis using histological verification or clinical follow-up as a reference standard. Multivariable logistic regression analysis was performed to determine pre-treatment predictors of interval metastasis. A prediction score was developed to predict the probability of interval metastasis.
Results: Of 783 patients that underwent 18F-FDG PET/CT restaging, 65 (8.3%) were found to have interval metastasis and 44 (5.6%) were deemed to have false positive lesions. The resulting sensitivity and specificity was 74.7% (95% CI: 64.3-83.4%) and 93.7% (95% CI: 91.6-95.4%), respectively. Multivariable analysis revealed that tumor length, cN status, squamous cell tumor histology, and baseline SUVmax were associated with interval metastasis. Based on these criteria, a prediction score was developed with an optimism adjusted C-index of 0.67 that demonstrated accurate calibration.
Conclusions: 18F-FDG PET/CT restaging detects distant interval metastases in 8.3% of patients after chemoradiotherapy for oesophageal cancer. The provided prediction score may stratify risk of developing interval metastasis, and could be used to prioritize additional restaging modalities for patients most likely to benefit.
Keywords: 18F-FDG PET/CT; Cancer staging; Chemoradiotherapy; Esophagectomy; Oesophageal cancer.
Conflict of interest statement
Conflict of interest
Steven H. Lin received research funding from STCube Pharmaceuticals, Genetech, Peregrine Pharmaceuticals, Hitachi Chemical, and honorarium from AstraZeneca. All other authors have no conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This retrospective study was approved by our Institutional Review Board, and the need for written informed consent was waived.
Figures

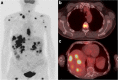



References
-
- Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. - DOI - PubMed
-
- Teoh AYB, Chiu PWY, Yeung WK, Liu SYW, Wong SKH, Ng EKW. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: results from a randomized controlled trial. Ann Oncol. 2013;24:165–171. doi: 10.1093/annonc/mds206. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

