The SERCA2: A Gatekeeper of Neuronal Calcium Homeostasis in the Brain
- PMID: 29663107
- PMCID: PMC11481958
- DOI: 10.1007/s10571-018-0583-8
The SERCA2: A Gatekeeper of Neuronal Calcium Homeostasis in the Brain
Abstract
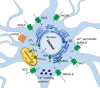
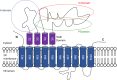
Calcium (Ca2+) ions are prominent cell signaling regulators that carry information for a variety of cellular processes and are critical for neuronal survival and function. Furthermore, Ca2+ acts as a prominent second messenger that modulates divergent intracellular cascades in the nerve cells. Therefore, nerve cells have developed intricate Ca2+ signaling pathways to couple the Ca2+ signal to their biochemical machinery. Notably, intracellular Ca2+ homeostasis greatly relies on the rapid redistribution of Ca2+ ions into the diverse subcellular organelles which serve as Ca2+ stores, including the endoplasmic reticulum (ER). It is well established that Ca2+ released into the neuronal cytoplasm is pumped back into the ER by the sarco-/ER Ca2+ ATPase 2 (SERCA2), a P-type ion-motive ATPase that resides on the ER membrane. Even though the SERCA2 is constitutively expressed in nerve cells, its precise role in brain physiology and pathophysiology is not well-characterized. Intriguingly, SERCA2-dependent Ca2+ dysregulation has been implicated in several disorders that affect cognitive function, including Darier's disease, schizophrenia, Alzheimer's disease, and cerebral ischemia. The current review summarizes knowledge on the expression pattern of the different SERCA2 isoforms in the nervous system, and further discusses evidence of SERCA2 dysregulation in various neuropsychiatric disorders. To the best of our knowledge, this is the first literature review that specifically highlights the critical role of the SERCA2 in the brain. Advancing knowledge on the role of SERCA2 in maintaining neuronal Ca2+ homeostasis may ultimately lead to the development of safer and more effective pharmacotherapies to combat debilitating neuropsychiatric disorders.
Keywords: Brain; Calcium; Darier’s disease; Neuron; SERCA2.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
ER-to-Golgi blockade of nascent desmosomal cadherins in SERCA2-inhibited keratinocytes: Implications for Darier's disease.Traffic. 2017 Apr;18(4):232-241. doi: 10.1111/tra.12470. Epub 2017 Feb 28. Traffic. 2017. PMID: 28156030 Free PMC article.
-
Activation of the androgen receptor alters the intracellular calcium response to glutamate in primary hippocampal neurons and modulates sarco/endoplasmic reticulum calcium ATPase 2 transcription.Neuroscience. 2007 Oct 12;149(1):155-64. doi: 10.1016/j.neuroscience.2007.06.054. Epub 2007 Jul 28. Neuroscience. 2007. PMID: 17870249
-
Endoplasmic reticulum stress-related deficits in calcium clearance promote neuronal dysfunction that is prevented by SERCA2 gene augmentation.Cell Rep Med. 2024 Dec 17;5(12):101839. doi: 10.1016/j.xcrm.2024.101839. Epub 2024 Nov 29. Cell Rep Med. 2024. PMID: 39615485 Free PMC article.
-
Markers of squamous cell carcinoma in sarco/endoplasmic reticulum Ca2+ ATPase 2 heterozygote mice keratinocytes.Prog Biophys Mol Biol. 2010 Sep;103(1):81-7. doi: 10.1016/j.pbiomolbio.2009.10.005. Epub 2009 Oct 17. Prog Biophys Mol Biol. 2010. PMID: 19840814 Review.
-
A Role for SERCA Pumps in the Neurobiology of Neuropsychiatric and Neurodegenerative Disorders.Adv Exp Med Biol. 2020;1131:131-161. doi: 10.1007/978-3-030-12457-1_6. Adv Exp Med Biol. 2020. PMID: 31646509 Review.
Cited by
-
Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis.Cells. 2019 Oct 10;8(10):1232. doi: 10.3390/cells8101232. Cells. 2019. PMID: 31658749 Free PMC article. Review.
-
Calcium Ions in the Physiology and Pathology of the Central Nervous System.Int J Mol Sci. 2024 Dec 6;25(23):13133. doi: 10.3390/ijms252313133. Int J Mol Sci. 2024. PMID: 39684844 Free PMC article. Review.
-
Axonal Endoplasmic Reticulum Dynamics and Its Roles in Neurodegeneration.Front Neurosci. 2020 Jan 29;14:48. doi: 10.3389/fnins.2020.00048. eCollection 2020. Front Neurosci. 2020. PMID: 32116502 Free PMC article. Review.
-
Patients with Darier Disease Exhibit Cognitive Impairment while Patients with Hailey-Hailey Disease Do Not: An Experimental, Matched Case-control Study.Acta Derm Venereol. 2021 Jun 22;101(6):adv00476. doi: 10.2340/00015555-3818. Acta Derm Venereol. 2021. PMID: 33928397 Free PMC article.
-
Linking Biochemical and Structural States of SERCA: Achievements, Challenges, and New Opportunities.Int J Mol Sci. 2020 Jun 10;21(11):4146. doi: 10.3390/ijms21114146. Int J Mol Sci. 2020. PMID: 32532023 Free PMC article. Review.
References
-
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I (2009) Amyloid-β as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci 12:1567 - PubMed
-
- Artola A, Singer W (1993) Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci 16:480–487 - PubMed
-
- Baba-Aissa F, Raeymaekers L, Wuytack F, De Greef C, Missiaen L, Casteels R (1996) Distribution of the organellar Ca2+ transport ATPase SERCA2 isoforms in the cat brain. Brain Res 743:141–153 - PubMed
-
- Baba-Aissa F, Raeymaekers L, Wuytack F, Dode L, Casteels R (1998) Distribution and isoform diversity of the organellar Ca2+ pumps in the brain. Mol Chem Neuropathol 33:199–208. 10.1007/bf02815182 - PubMed
-
- Baker K, Edwards T, Rickard N (2008) Inhibition of mGluR1 and IP 3 Rs impairs long-term memory formation in young chicks. Neurobiol Learn Mem 90:269–274 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

