A review of studies evaluating the effectiveness of risk minimisation measures in Europe using the European Union electronic Register of Post-Authorization Studies
- PMID: 29663572
- PMCID: PMC6055865
- DOI: 10.1002/pds.4434
A review of studies evaluating the effectiveness of risk minimisation measures in Europe using the European Union electronic Register of Post-Authorization Studies
Abstract
Purpose: An important element of risk management is the planning and implementation of risk minimisation measures (RMMs) and the evaluation of their effectiveness by process or outcome indicators. The aim of this review is to summarize the characteristics of risk minimisation (RM) effectiveness studies in Europe and provide an overview of RMMs and their effectiveness.
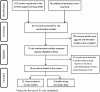
Methods: This was a qualitative review of RM effectiveness studies in the European Union electronic Register of Post-Authorization Studies (EU PAS Register); data extracted included study design, population, sample size, data sources, drug information, RMMs, study period, indicators, and their reported effectiveness.
Results: Of the 872 records in the EU PAS Register, 19 studies evaluating the effectiveness of RMMs were included. Eleven were cross-sectional surveys and 8 used secondary data sources. Eighty-nine percent (17/19) evaluated additional RMMs (used when routine RMMs are considered insufficient), and 36% (7/19) evaluated changes in routine RMMs (applicable to all medicinal products). A total of 42 effectiveness indicators were identified: 18 process and 24 outcomes. Half of the indicators (21/42) were successful; 2% (1/42) indicators were partially successful; 17% (7/42) indicators were inconclusive. Effectiveness of the remaining 31% (13/42) indicators could not be determined owing to limited information. The United Kingdom was the most frequent country for the conduct of RM effectiveness studies.
Conclusions: Most of the included studies evaluated additional RMMs. Half of the effectiveness indicators (process and/or outcome) were reported as successful. This review provides evidence to support the development of future guidance on the effectiveness of RM in Europe.
Keywords: Europe; medical records; pharmacoepidemiology; review; risk management; surveys and questionnaires.
© 2018 The Authors. Pharmacoepidemiology & Drug Safety Published by John Wiley & Sons Ltd.
References
-
- EMA . Good pharmacovigilance practices—European Medicines Agency. http://www.ema/ema/index.jsp?curl=pages/regulation/document_listing/docu.... Accessed February 15, 2017.
-
- EMA . Pharmacovigilance legislation—European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general.... Accessed February 15, 2017.
-
- Council for International Organizations of Medical Sciences (CIOMS). Chapter V. Evaluating effectiveness of risk minimisation. Practical approaches to risk minimisation for medicinal products: report of CIOMS Working Group IX. Vol 2015: Geneva: Council for International Organizations of medical Sciences; 2014.
-
- EMA . Guideline on good pharmacovigilance practices (GVP) Module XVI—risk minimisation measures: selection of tools and effectiveness indicators (Rev 1). 2014; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed February 15, 2017.
-
- ENCePP activity report 2014. EMA; http://www.encepp.eu/publications/documents/ENCePPActivityReport2014.pdf. Accessed February 15, 2017.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical