Water Formation under Silica Thin Films: Real-Time Observation of a Chemical Reaction in a Physically Confined Space
- PMID: 29663598
- PMCID: PMC6055755
- DOI: 10.1002/anie.201802000
Water Formation under Silica Thin Films: Real-Time Observation of a Chemical Reaction in a Physically Confined Space
Abstract
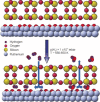
Using low-energy electron microscopy and local photoelectron spectroscopy, water formation from adsorbed O and H2 on a Ru(0001) surface covered with a vitreous SiO2 bilayer (BL) was investigated and compared to the same reaction on bare Ru(0001). In both cases the reaction is characterized by moving reaction fronts. The reason for this might be related to the requirement of site release by O adatoms for further H2 -dissociative adsorption. Apparent activation energies (Eaapp ) are found for the front motion of 0.59 eV without cover and 0.27 eV under cover. We suggest that the smaller activation energy but higher reaction temperature for the reaction on the SiO2 BL covered Ru(0001) surface is due to a change of the rate-determining step. Other possible effects of the cover are discussed. Our results give the first values for Eaapp in confined space.
Keywords: LEEM; confined chemistry; heterogeneous catalysis; hydrogen oxidation; model systems.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- None
-
- Emmez E., Anibal Boscoboinik J., Tenney S., Sutter P., Shaikhutdinov S., Freund H.-J., Surf. Sci. 2016, 646, 19–25;
-
- Emmez E., Yang B., Shaikhutdinov S., Freund H. J., J. Phys. Chem. C 2014, 118, 29034–29042.
-
- Miners S. A., Rance G. A., Khlobystov A. N., Chem. Commun. 2013, 49, 5586–5588. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

