Patient-Specific Bioinks for 3D Bioprinting of Tissue Engineering Scaffolds
- PMID: 29663706
- PMCID: PMC6422175
- DOI: 10.1002/adhm.201701347
Patient-Specific Bioinks for 3D Bioprinting of Tissue Engineering Scaffolds
Abstract
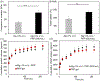
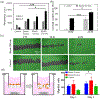
Bioprinting has emerged as a promising tool in tissue engineering and regenerative medicine. Various 3D printing strategies have been developed to enable bioprinting of various biopolymers and hydrogels. However, the incorporation of biological factors has not been well explored. As the importance of personalized medicine is becoming more clear, the need for the development of bioinks containing autologous/patient-specific biological factors for tissue engineering applications becomes more evident. Platelet-rich plasma (PRP) is used as a patient-specific source of autologous growth factors that can be easily incorporated to hydrogels and printed into 3D constructs. PRP contains a cocktail of growth factors enhancing angiogenesis, stem cell recruitment, and tissue regeneration. Here, the development of an alginate-based bioink that can be printed and crosslinked upon implantation through exposure to native calcium ions is reported. This platform can be used for the controlled release of PRP-associated growth factors which may ultimately enhance vascularization and stem cell migration.
Keywords: 3D bioprinting; alginate-based hydrogels; patient-specific bioinks; platelet-rich plasma (PRP); tissue engineering.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Ferris CJ, Gilmore KJ, Beirne S, McCallum D, Wallace GG, Biomaterials Science 2013, 1, 224. - PubMed
-
- Drury JL, Mooney DJ, Biomaterials 2003, 24, 4337; - PubMed
- Tibbitt MW, Anseth KS, Biotechnology and bioengineering 2009, 103, 655; - PMC - PubMed
- Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, Camci‐Unal G, Dokmeci MR, Peppas NA, Khademhosseini A, Advanced Materials 2014, 26, 85; - PMC - PubMed
- Fallahi A, Khademhosseini A, Tamayol A, Trends in biotechnology 2016, 34, 683. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

