Cedrus deodara: In vitro antileishmanial efficacy & immumomodulatory activity
- PMID: 29664038
- PMCID: PMC5926351
- DOI: 10.4103/ijmr.IJMR_959_16
Cedrus deodara: In vitro antileishmanial efficacy & immumomodulatory activity
Abstract
Background & objectives: The existing antileishmanial drugs for complete cure of visceral leishmaniasis (kala-azar) are limited. The available drugs are either toxic or less effective leading to disease relapse or conversion to post-kala-azar dermal leishmaniasis. Several herbal extracts have been shown to have antileishmanial activity, but a herbal drug may not always be safe. In the present study, the extract of Cedrus deodara leaves has been standardized and tested for immunomodulatory antileishmanial activities.
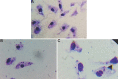
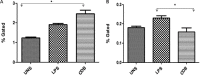
Methods: The extracts of C. deodara leaves with different solvents such as benzene, chloroform, ethyl acetate and methanol were made by soxhlation process. Solvents were removed under reduced pressure and temperature using rotary evaporator. The antileishmanial bioassay test was performed with in vitro maintained parasites. Immunomodulatory activity of different extracts was tested by flow cytometry. Standardization of the effective fraction was performed with Linalool as a marker compound through reverse-phase high-performance liquid chromatography.
Results: The extract with the use of benzene solvent showed strong antileishmanial activities within a dose 25-200 μg/ml culture with non-significant haemolytic activities and significant immunomodulant activities against the host cells. Linalool was found to be 1.29 per cent in the effective extract of C. deodara.
Interpretation & conclusions: The antileishmanial activity of C. deodara, as assessed by bioassay testing on.
Leishmania donovani: parasites and immunomodulatory effect of benzene extract of leaves on host cells indicated that it might be a potential new safe therapeutic target to cure the visceral leishmaniasis.
Keywords: Arginase - Cedrus deodara - linalool - reverse-phase high-performance liquid chromatography - visceral leishmaniasis.
Conflict of interest statement
None
Figures






References
-
- World Health Organization. World Health Report. Geneva: WHO; 2012. pp. 186–91.
-
- Gupta A, Nayar M, Mishra SS, Lahariya C. Visceral leishmaniasis (Kala azar) elimination from Indian sub continents by 2015. Int J Trop Dis Health. 2013;32:73–81.
-
- Das VN, Pandey K, Verma N, Lal CS, Bimal S, Topno RK, et al. Short report: Development of post-Kala-Azar dermal leishmaniasis (PKDL) in miltefosine-treated visceral leishmaniasis. Am J Trop Med Hyg. 2009;80:336–8. - PubMed
-
- Thakur CP, Narayan S. A comparative evaluation of amphotericin B and sodium antimony gluconate, as first-line drugs in the treatment of Indian visceral leishmaniasis. Ann Trop Med Parasitol. 2004;98:129–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
