Catalytic Enantioselective Dihalogenation in Total Synthesis
- PMID: 29664281
- PMCID: PMC5987034
- DOI: 10.1021/acs.accounts.8b00064
Catalytic Enantioselective Dihalogenation in Total Synthesis
Abstract
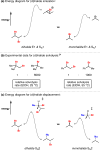
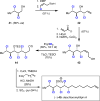
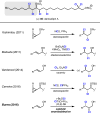
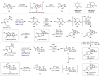
To date, more than 5000 biogenic halogenated molecules have been characterized. This number continues to increase as chemists explore chloride- and bromide-rich marine environments in search of novel bioactive natural products. Naturally occurring organohalogens span nearly all biosynthetic structural classes, exhibit a range of unique biological activities, and have been the subject of numerous investigations. Despite the abundance of and interest in halogenated molecules, enantioselective methods capable of forging carbon-halogen bonds in synthetically relevant contexts remain scarce. Accordingly, syntheses of organohalogens often rely on multistep functional group interconversions to establish carbon-halogen stereocenters. Our group has developed an enantioselective dihalogenation reaction and utilized it in the only reported examples of catalytic enantioselective halogenation in natural product synthesis. In this Account, we describe our laboratory's development of a method for catalytic, enantioselective dihalogenation and the application of this method to the synthesis of both mono- and polyhalogenated natural products. In the first part, we describe the initial discovery of a TADDOL-mediated dibromination of cinnamyl alcohols. Extension of this reaction to a second-generation system capable of selective bromochlorination, dichlorination, and dibromination is then detailed. This system is capable of controlling the enantioselectivity of dihalide formation, chemoselectivity for polyolefinic substrates, and regioselectivity in the case of bromochlorination. The ability of this method to exert control over regioselectivity of halide delivery permits selective halogenation of electronically nonbiased olefins required for total synthesis. In the second part, we demonstrate how the described dihalogenation has provided efficient access to a host of structurally diverse natural products. The most direct application of this methodology is in the synthesis of naturally occurring vicinal dihalides. Chiral vicinal bromochlorides represent a class of >175 natural products; syntheses of five members of this class, including its flagship member, (+)-halomon, have been accomplished through use of the catalytic, enantioselective bromochlorination. Likewise, enantioselective dichlorination has provided selective access to two members of the chlorosulfolipids, a class of linear, acyclic polychlorides. Synthesis of chiral monohalides has been achieved through solvolysis of enantioenriched bromochlorides; this approach has resulted in the synthesis of five bromocyclohexane-containing natural products through an enantiospecific bromopolyene cyclization. In reviewing these syntheses, a framework for the synthesis of chiral organohalogens mediated by catalytic, enantioselective dihalogenation has emerged. The development of a selective dihalogenation method has been highly enabling in the synthesis of halogenated natural products. In this Account, we detail all examples of catalytic, enantioselective halogenation in total synthesis and encourage the further development of synthetically useful halogenation methodologies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
-
- Gribble GW. Naturally Occurring Organohalogen Compounds–A Comprehensive Survey. Springer-Verlag; Wien, Austria: 1996. - PubMed
- Gribble GW. Naturally Occurring Organohalogen Compounds–A Comprehensive Update. Springer-Verlag; Wien Austria: 2010.
-
- Chung W-j, Vanderwal CD. Stereoselective Halogenation in Natural Product Synthesis. Angew. Chem. Int. Ed. 2016;55:4396–4434. - PMC - PubMed
- Cheng YA, Yu WZ, Yeung Y-Y. Recent Advances in Asymmetric Intra- and Intermolecular Halofunctionalizations of Alkenes. Org. Biomol. Chem. 2014;12:2333–2343. - PubMed
- Chen J, Zhou L. Recent Progress in the Asymmetric Intermolecular Halogenation of Alkenes. Synthesis. 2014;46:586–595.
- Tan CK, Yeung Y-Y. Recent Advances in Stereoselective Bromofunctionalization of Alkenes Using N-Bromoamide Reagents. Chem. Commun. 2013;49:7985–7996. - PubMed
-
- Snyder SA, Tang Z-Y, Gupta R. Enantioselective Total Synthesis of (−)-Napyradiomycin A1 via Asymmetric Chlorination of an Isolated Olefin. J. Am. Chem. Soc. 2009;131:5744–5745. - PubMed
-
- Denmark SE, Kuester WE, Burk MT. Catalytic, Asymmetric Halofunctionalization of Alkenes–A Critical Perspective. Angew. Chem. Int. Ed. 2012;51:10938–10953. - PMC - PubMed
- Cresswell AJ, Eey ST-C, Denmark SE. Catalytic, Stereoselective Dihalogenation of Alkenes: Challenges and Opportunities. Angew. Chem. Int. Ed. 2015;54:15642–15682. - PMC - PubMed
-
- Denmark SE, Burk MT, Hoover AJ. On the Absolute Configurational Stability of Bromonium and Chloronium Ions. J. Am. Chem. Soc. 2010;132:1232–1233. - PubMed
- Brown RS. Investigation of the Early Steps in Electrophilic Bromination through the Study of the Reaction with Sterically Encumbered Olefins. Acc. Chem. Res. 1997;30:131–137.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

