Rat anterior cingulate cortex recalls features of remote reward locations after disfavoured reinforcements
- PMID: 29664400
- PMCID: PMC5931797
- DOI: 10.7554/eLife.29793
Rat anterior cingulate cortex recalls features of remote reward locations after disfavoured reinforcements
Abstract
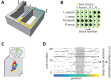
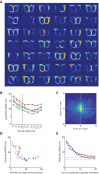
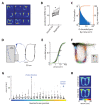
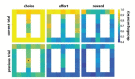
The anterior cingulate cortex (ACC) encodes information supporting mnemonic and cognitive processes. We show here that a rat's position can be decoded with high spatiotemporal resolution from ACC activity. ACC neurons encoded the current state of the animal and task, except for brief excursions that sometimes occurred at target feeders. During excursions, the decoded position became more similar to a remote target feeder than the rat's physical position. Excursions recruited activation of neurons encoding choice and reward, and the likelihood of excursions at a feeder was inversely correlated with feeder preference. These data suggest that the excursion phenomenon was related to evaluating real or fictive choice outcomes, particularly after disfavoured reinforcements. We propose that the multiplexing of position with choice-related information forms a mental model isomorphic with the task space, which can be mentally navigated via excursions to recall multimodal information about the utility of remote locations.
Keywords: choice; cognitive map; decoding; ensemble activity; neural network; neuroscience; prospection; rat.
© 2018, Mashhoori et al.
Conflict of interest statement
AM, SH, BM, DE, AG No competing interests declared
Figures


References
-
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52:336–372. doi: 10.1016/j.geb.2004.06.010. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

