Finite-Size Effects of Binary Mutual Diffusion Coefficients from Molecular Dynamics
- PMID: 29664633
- PMCID: PMC5943679
- DOI: 10.1021/acs.jctc.8b00170
Finite-Size Effects of Binary Mutual Diffusion Coefficients from Molecular Dynamics
Abstract
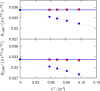
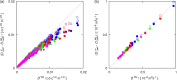
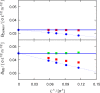
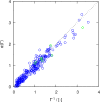
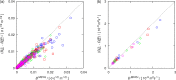
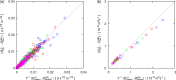
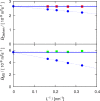
Molecular dynamics simulations were performed for the prediction of the finite-size effects of Maxwell-Stefan diffusion coefficients of molecular mixtures and a wide variety of binary Lennard-Jones systems. A strong dependency of computed diffusivities on the system size was observed. Computed diffusivities were found to increase with the number of molecules. We propose a correction for the extrapolation of Maxwell-Stefan diffusion coefficients to the thermodynamic limit, based on the study by Yeh and Hummer ( J. Phys. Chem. B , 2004 , 108 , 15873 - 15879 ). The proposed correction is a function of the viscosity of the system, the size of the simulation box, and the thermodynamic factor, which is a measure for the nonideality of the mixture. Verification is carried out for more than 200 distinct binary Lennard-Jones systems, as well as 9 binary systems of methanol, water, ethanol, acetone, methylamine, and carbon tetrachloride. Significant deviations between finite-size Maxwell-Stefan diffusivities and the corresponding diffusivities at the thermodynamic limit were found for mixtures close to demixing. In these cases, the finite-size correction can be even larger than the simulated (finite-size) Maxwell-Stefan diffusivity. Our results show that considering these finite-size effects is crucial and that the suggested correction allows for reliable computations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Kohl A. L.; Nielsen R.. Gas Purification, 5th ed.; Gulf Professional Publishing: Houston, 1997.
-
- Lyons W.; Plisga G. J.; Lorenz M.. Standard Handbook of Petroleum and Natural Gas Engineering, 3rd ed.; Gulf Professional Publishing: Waltham, 2015.
-
- Economou I.; Krokidas P.; Michalis V.; Moultos O.; Tsimpanogiannis I.; Vergadou N.. The Water-Food-Energy Nexus: Processes, Technologies, and Challenges; CRC Press: Boca Raton, 2017; Chapter 13, pp 633–660.
-
- Economou I. G.; de Hemptinne J.-C.; Dohrn R.; Hendriks E.; Keskinen K.; Baudouin O. Industrial Use of Thermodynamics Workshop: Round Table Discussion on 8 July 2014. Chem. Eng. Res. Des. 2014, 92, 2795–2796. 10.1016/j.cherd.2014.10.022. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

