Pharmacological induction of hypoxia-inducible transcription factor ARNT attenuates chronic kidney failure
- PMID: 29664738
- PMCID: PMC6025987
- DOI: 10.1172/JCI89632
Pharmacological induction of hypoxia-inducible transcription factor ARNT attenuates chronic kidney failure
Abstract
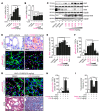
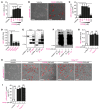
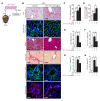
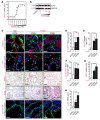
Progression of chronic kidney disease associated with progressive fibrosis and impaired tubular epithelial regeneration is still an unmet biomedical challenge because, once chronic lesions have manifested, no effective therapies are available as of yet for clinical use. Prompted by various studies across multiple organs demonstrating that preconditioning regimens to induce endogenous regenerative mechanisms protect various organs from later incurring acute injuries, we here aimed to gain insights into the molecular mechanisms underlying successful protection and to explore whether such pathways could be utilized to inhibit progression of chronic organ injury. We identified a protective mechanism controlled by the transcription factor ARNT that effectively inhibits progression of chronic kidney injury by transcriptional induction of ALK3, the principal mediator of antifibrotic and proregenerative bone morphogenetic protein-signaling (BMP-signaling) responses. We further report that ARNT expression itself is controlled by the FKBP12/YY1 transcriptional repressor complex and that disruption of such FKBP12/YY1 complexes by picomolar FK506 at subimmunosuppressive doses increases ARNT expression, subsequently leading to homodimeric ARNT-induced ALK3 transcription. Direct targeting of FKBP12/YY1 with in vivo morpholino approaches or small molecule inhibitors, including GPI-1046, was equally effective for inducing ARNT expression, with subsequent activation of ALK3-dependent canonical BMP-signaling responses and attenuated chronic organ failure in models of chronic kidney disease, and also cardiac and liver injuries. In summary, we report an organ-protective mechanism that can be pharmacologically modulated by immunophilin ligands FK506 and GPI-1046 or therapeutically targeted by in vivo morpholino approaches.
Keywords: Chronic kidney disease; Nephrology.
Conflict of interest statement
Figures









Comment in
-
Targeting ARNT to attenuate renal fibrosis.Nat Rev Nephrol. 2018 Sep;14(9):535. doi: 10.1038/s41581-018-0038-y. Nat Rev Nephrol. 2018. PMID: 29955104 No abstract available.
-
ARNT as a Novel Antifibrotic Target in CKD.Am J Kidney Dis. 2019 Feb;73(2):281-284. doi: 10.1053/j.ajkd.2018.08.009. Epub 2018 Oct 18. Am J Kidney Dis. 2019. PMID: 30343956 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

