Cofilin-2 Acts as a Marker for Predicting Radiotherapy Response and Is a Potential Therapeutic Target in Nasopharyngeal Carcinoma
- PMID: 29664897
- PMCID: PMC5921956
- DOI: 10.12659/msm.909832
Cofilin-2 Acts as a Marker for Predicting Radiotherapy Response and Is a Potential Therapeutic Target in Nasopharyngeal Carcinoma
Abstract
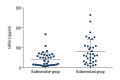
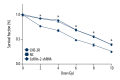
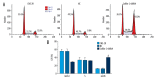
BACKGROUND The purpose of this study was to determine whether cofilin-2 could serve as a protein marker for predicting radiotherapy response and as a potential therapeutic target in nasopharyngeal carcinoma (NPC). MATERIAL AND METHODS Cofilin-2 protein levels in serum and tissue samples from patients with NPC were assessed by sandwich ELISA and IHC. In vitro, cofilin-2 levels in CNE-2R cells were significantly higher than those of CNE-2 cells. Meanwhile, CNE-2R cells were silenced for cofilin-2 to obtain a stable cofilin-2-RNAi-LV3 cell line. Then, cell proliferation, radiosensitivity, invasion and migration abilities, cell cycle, and apoptosis were evaluated by Cell Counting Kit 8 assay (CCK-8), flow cytometry (FCM), clone formation assay, and in vitro. RESULTS The secreted levels of the cofilin-2 protein in radioresistant NPC patients were significantly higher than those of radiosensitive cases. After cofilin-2 knockdown in nasopharyngeal carcinoma CNE-2R cells, proliferation was decreased, while apoptosis and radiosensitivity were enhanced; cell cycle distribution was altered, and the transplanted tumors in nude mice grew significantly less. CONCLUSIONS Overall, our findings suggest that cofilin-2 acts as a marker for predicting radiotherapy response and is a potential therapeutic target in nasopharyngeal carcinoma.
Conflict of interest statement
None.
Figures






References
-
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66(2):115–32. - PubMed
-
- Tang LL, Chen WQ, Xue WQ, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374(1):22–30. - PubMed
-
- Leung SF, Teo PM, Shiu WW, et al. Clinical features and management of distant metastases of nasopharyngeal carcinoma. J Otolaryngol. 1991;20(1):27–29. - PubMed
-
- Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: Overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23(2):261–70. - PubMed
-
- Li ZQ, Xia YF, Liu Q, et al. Radiotherapy-related typing in 842 patients in canton with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):1011–16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

