Metabolic Features of Nonalcoholic Fatty Liver (NAFL) in Obese Adolescents: Findings From a Multiethnic Cohort
- PMID: 29665034
- PMCID: PMC6173637
- DOI: 10.1002/hep.30035
Metabolic Features of Nonalcoholic Fatty Liver (NAFL) in Obese Adolescents: Findings From a Multiethnic Cohort
Abstract
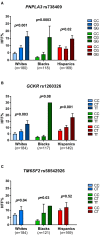
We conducted a prospective study in a large, multiethnic cohort of obese adolescents to characterize clinical and genetic features associated with pediatric nonalcoholic fatty liver (NAFL), the most common cause of chronic liver disease in youth. A total of 503 obese adolescents were enrolled, including 191 (38.0%) whites, 134 (26.6%) blacks, and 178 (35.4%) Hispanics. Participants underwent abdominal magnetic resonance imaging (MRI) to quantify hepatic fat fraction (HFF), an oral glucose tolerance test (OGTT) to assess glucose tolerance and insulin sensitivity, and the genotyping of three single-nucleotide polymorphisms (SNPs) associated with nonalcoholic fatty liver disease (NAFLD) (patatin-like phospholipase domain-containing protein 3 [PNPLA3] rs738409, glucokinase regulatory protein [GCKR] rs1260326, and transmembrane 6 superfamily member 2 [TM6SF2] rs58542926). Assessments were repeated in 133 subjects after a 2-year follow-up. Prevalence of nonalcoholic fatty liver (NAFL) was 41.6% (209 patients) and ranged widely among ethnicities, being 42.9% in whites, 15.7% in blacks, and 59.6% in Hispanics (P < 0.0001). Among adolescents with NAFL, blacks showed the highest prevalence of altered glucose homeostasis (66%; P = 0.0003). Risk factors for NAFL incidence were white or Hispanic ethnicity (P = 0.021), high fasting C-peptide levels (P = 0.0006), and weight gain (P = 0.0006), whereas baseline HFF (P = 0.004) and weight loss (P = 0.032) predicted resolution of NAFL at follow-up. Adding either gene variant to these variables improved significantly the model predictive performance.
Conclusion: Black obese adolescents are relatively protected from liver steatosis, but are more susceptible to the deleterious effects of NAFL on glucose metabolism. The combination of ethnicity/race with markers of insulin resistance and genetic factors might help identify obese youth at risk for developing NAFL.
Trial registration: ClinicalTrials.gov NCT01966627.
© 2018 The Authors. Hepatology published by Wiley Periodicals, Inc. on behalf of American Association for the Study of Liver Diseases.
Figures

Comment in
-
Nonalcoholic fatty liver disease in obese adolescents: the role of genetic polymorphisms.Hepatobiliary Surg Nutr. 2019 Apr;8(2):179-180. doi: 10.21037/hbsn.2018.12.03. Hepatobiliary Surg Nutr. 2019. PMID: 31098374 Free PMC article. No abstract available.
-
Paediatric non-alcoholic fatty liver disease: a more complex disease than in the adulthood?Hepatobiliary Surg Nutr. 2019 Jun;8(3):270-273. doi: 10.21037/hbsn.2018.12.16. Hepatobiliary Surg Nutr. 2019. PMID: 31245411 Free PMC article. No abstract available.
References
-
- Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388‐1393. - PubMed
-
- Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers 2015;1:15080. - PubMed
-
- Nobili V, Alisi A, Grimaldi C, Liccardo D, Francalanci P, Monti L, et al. Non‐alcoholic fatty liver disease and hepatocellular carcinoma in a 7‐year‐old obese boy: coincidence or comorbidity? Pediatr Obes 2014;9:e99‐e102. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

