Phase 1 studies to assess the safety, tolerability and pharmacokinetics of JTE-052 (a novel Janus kinase inhibitor) ointment in Japanese healthy volunteers and patients with atopic dermatitis
- PMID: 29665062
- PMCID: PMC6001687
- DOI: 10.1111/1346-8138.14322
Phase 1 studies to assess the safety, tolerability and pharmacokinetics of JTE-052 (a novel Janus kinase inhibitor) ointment in Japanese healthy volunteers and patients with atopic dermatitis
Abstract
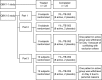
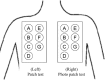
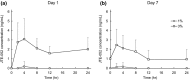
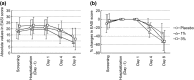
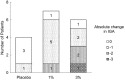
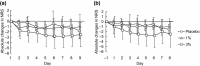
The purpose of the present two phase 1 studies was to assess the safety, tolerability and pharmacokinetics for topical application of a novel Janus kinase (JAK) inhibitor, JTE-052, in Japanese healthy adult male volunteers and Japanese adult patients with atopic dermatitis (AD). Additionally, exploratory investigation was performed on the efficacy for disease severity and pruritus score in AD patients. In the QBX1-1 study, the cutaneous safety of JTE-052 ointment by a patch test and a photo patch test was assessed in an intra-individual comparative study using placebo ointment, white petrolatum and non-application as comparators. The study demonstrated that JTE-052 ointment would be associated with a low potential for phototoxicity but had no potential for skin irritation or photoallergy. In the QBX1-2 study, it was revealed that the systemic exposure to JTE-052 in both healthy volunteers with normal skin and AD patients with inflamed skin was low in application of not only 1% but also 3% JTE-052 ointment. JTE-052 ointments of 1% and 3% were generally safe and well tolerated in both populations. In a repeated twice-daily application for 7 days, the efficacy of JTE-052 ointment to AD patients was observed with both 1% and 3% ointments in the exploratory investigations evaluated by Eczema Area and Severity Index, Investigator's Global Assessment and Numeric Rating Scale assessments. The mean scores for each assessment declined from the baseline throughout the study. These results suggest that the treatment of JTE-052 ointment is generally safe and effective in AD patients, although further large confirmatory studies are needed.
Keywords: JTE-052; atopic dermatitis; patch test; pharmacokinetics; topical.
© 2018 Japan Tobacco Inc. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Figures
References
-
- Bieber T. Atopic dermatitis. N Engl J Med 2008; 358: 1483–1494. - PubMed
-
- Saeki H, Nakahara T, Tanaka A et al Clinical practice guidelines for the management of atopic dermatitis 2016. J Dermatol 2016; 43: 1117–1145. - PubMed
-
- Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol 2005; 22: 192–199. - PubMed
-
- Japanese FK 506 Ointment Study Group . Clinical guidance for treatment of patients with atopic dermatitis by tacrolimus ointment 0.1% and 0.03%. Jpn J Clin Dermatol 2003; 57: 1217–1234 (In Japanese).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

