Cervical cancer - State of the science: From angiogenesis blockade to checkpoint inhibition
- PMID: 29666026
- PMCID: PMC6720107
- DOI: 10.1016/j.ygyno.2018.01.009
Cervical cancer - State of the science: From angiogenesis blockade to checkpoint inhibition
Abstract
Vascular endothelial growth factor (VEGF) has emerged as a therapeutic target in several malignancies, including cervical cancer. Chemotherapy doublets combined with the fully humanized monoclonal antibody, bevacizumab, now constitute first-line therapy for women struggling with recurrent/metastatic cervical carcinoma. Regulatory approval for this indication was based on the phase III randomized trial, GOG 240, which demonstrated a statistically significant and clinically meaningful improvement in overall survival when bevacizumab was added to chemotherapy: 17.0 vs 13.3 months; HR 0.71; 98% CI, 0.54-0.95; p = .004. Incorporation of bevacizumab resulted in significant improvements in progression-free survival and response. These benefits were not accompanied by deterioration in quality of life. GOG 240 identified vaginal fistula as a new adverse event associated with bevacizumab use. All fistulas occurred in women who had received prior pelvic radiotherapy, and none resulted in emergency surgery, sepsis, or death. Final protocol-specified analysis demonstrated continued separation of the survival curves favoring VEGF inhibition: 16.8 vs 13.3 months; HR 0.77; 95% CI, 0.62-9.95; p = .007. Post-progression survival was not significantly different between the arms in GOG 240. Moving forward, immunotherapy has now entered the clinical trial arena to address the high unmet clinical need for effective and tolerable second line therapies in this patient population. Targeting the programmed cell death 1/programmed death ligand 1 (PD-1/PD-L1) pathway using checkpoint inhibitors to break immunologic tolerance is promising. The immunologic landscape involving human papillomavirus-positive head and neck carcinoma and cutaneous squamous cell carcinoma can be informative when considering feasibility of checkpoint blockade in advanced cervical cancer. Phase II studies using anti-PD-1 molecules, nivolumab and pembrolizumab are ongoing, and GOG 3016, the first phase III randomized trial of a checkpoint inhibitor (cemiplimab) in cervical cancer, recently activated. Important considerations in attempts to inhibit the inhibitors include pseudoprogression and post-progression survival, abscopal effects, and immune-related adverse events, including endocrinopathies.
Keywords: Advanced cervical cancer; Anti-angiogenesis therapy; Bevacizumab; Checkpoint inhibitors; Metastatic cervical cancer; Recurrent cervical cancer.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest statement
Dr. Lindsey Minion has nothing to disclose in relation to this manuscript. Dr. Tewari reports that his institution has received research grants from Genentech, and that he is on the Speaker’s Bureau for Merck and Roche. Dr. Tewari has also participated on an Advisory Board for Regeneron in 2017 and participated on two Advisory Board.
Figures
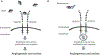



References
-
- Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA Cancer J. Clin 68 (2018)7–30. - PubMed
-
- Torre LA, Bray F, Siegel RL, et al., Global cancer statistics, 2012, CA Cancer J. Clin. 65 (2) (March 2015) 87–108. - PubMed
-
- Center for Disease Control and Prevention, HPV-associated cancers, http://www.cdc.gov/cancer/hpv/statistics/age.htm, Accessed date: 15 May 2017.
-
- Waggnor SE, Darcy KM, Tian C, Lanciano R, Smoking behavior in women with locally advanced cervical carcinoma: a Gynecologic Oncology Group study, Am. J.Obstet. Gynecol. 202 (3) (March 2010) (283.e1–7). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

