Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion
- PMID: 29666112
- PMCID: PMC5992863
- DOI: 10.1182/blood-2017-12-822619
Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion
Abstract
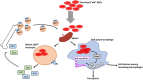
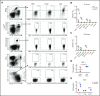
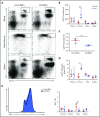
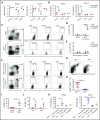
Macrophages play important roles in recycling iron derived from the clearance of red blood cells (RBCs). They are also a critically important component of host defense, protecting against invading pathogens. However, the effects on macrophage biology of acutely ingesting large numbers of RBCs are not completely understood. To investigate this issue, we used a mouse model of RBC transfusion and clearance, which mimics the clinical setting. In this model, transfusions of refrigerator storage-damaged (ie, "old") RBCs led to increased erythrophagocytosis by splenic red pulp macrophages (RPMs). This robust erythrophagocytosis induced ferroptosis, an iron-dependent form of cell death, in RPMs. This was accompanied by increases in reactive oxygen species and lipid peroxidation in vivo, which were reduced by treatment in vitro with ferrostatin-1, a ferroptosis inhibitor. Old RBC transfusions also induced RPM-dependent chemokine expression by splenic Ly6Chi monocytes, which signaled Ly6Chi monocyte migration from bone marrow to spleen, where these cells subsequently differentiated into RPMs. The combination of cell division among remaining splenic RPMs, along with the influx of bone marrow-derived Ly6Chi monocytes, suggests that, following RPM depletion induced by robust erythrophagocytosis, there is a coordinated effort to restore homeostasis of the RPM population by local self-maintenance and contributions from circulating monocytes. In conclusion, these findings may be clinically relevant to pathological conditions that can arise as a result of increased erythrophagocytosis, such as transfusion-related immunomodulation and impaired host immunity.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



References
-
- Luzzatto L, Nannelli C, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Hematol Oncol Clin North Am. 2016;30(2):373-393. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

