Genetics of diffuse large B-cell lymphoma
- PMID: 29666115
- PMCID: PMC5969374
- DOI: 10.1182/blood-2017-11-764332
Genetics of diffuse large B-cell lymphoma
Abstract
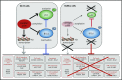
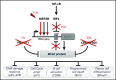
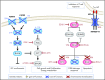
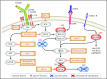
Diffuse large B-cell lymphoma (DLBCL), the most frequent subtype of lymphoid malignancy, remains a significant clinical challenge, as ∼30% of patients are not cured. Over the past decade, remarkable progress has been made in the understanding of the pathogenesis of this disease, spurred by the implementation of powerful genomic technologies that enabled the definition of its genetic and epigenetic landscape. These studies have uncovered a multitude of genomic alterations that contribute to the initiation and maintenance of the tumor clone by disrupting biological functions known to be critical for the normal biology of its cells of origin, germinal center B cells. The identified alterations involve epigenetic remodeling, block of differentiation, escape from immune surveillance, and the constitutive activation of several signal transduction pathways. This wealth of new information offers unique opportunities for the development of improved diagnostic and prognostic tools that could help guide the clinical management of DLBCL patients. Furthermore, a number of the mutated genes identified are potentially actionable targets that are currently being explored for the development of novel therapeutic strategies. This review summarizes current knowledge of the most common genetic alterations associated with DLBCL in relation to their functional impact on the malignant transformation process, and discusses their clinical implications for mechanism-based therapeutics.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer (IARC); 2016.
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. - PubMed
-
- Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14(8):517-534. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

