The French prospective multisite registry on sudden unexpected infant death (OMIN): rationale and study protocol
- PMID: 29666137
- PMCID: PMC5905759
- DOI: 10.1136/bmjopen-2017-020883
The French prospective multisite registry on sudden unexpected infant death (OMIN): rationale and study protocol
Abstract
Introduction: Even after 'back-to-sleep' campaigns, sudden unexpected infant death (SUID) continues to be the leading cause of death for infants 1 month to 1 year old in developed countries, with devastating social, psychological and legal implications for families. To sustainably tackle this problem and decrease the number of SUIDs, a French SUID registry was initiated in 2015 to (1) inform prevention with standardised data, (2) understand the mechanisms leading to SUID and the contribution of the already known or newly suggested risk factors and (3) gather a multidisciplinary group of experts to coordinate and develop innovative and urgent research in the SUID area.
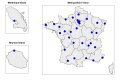
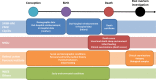
Methods and analysis: This observational multisite prospective observatory includes all cases of sudden unexpected deaths in children younger than 2 years occurring in the French territory covered by the 35 participating French referral centres. From these cases, various data concerning sociodemographic conditions, death scene, personal and family medical history, parental behaviours, sleep environment, clinical examinations, biological and imagery investigations and autopsy are systematically collected. These data will be complemented as of 2018 with a biobank of diverse biological samples (blood, hair, urine, faeces and cerebrospinal fluid), with other administrative health-related data (health claim reimbursements and hospital admissions) and socioenvironmental data. Insights from exploratory descriptive statistics and thematic analysis will be combined for the design of targeted strategies to effectively reduce preventable infant deaths.
Ethics and dissemination: The French sudden unexpected infant death registry (Observatoire National des Morts Inattendues du Nourrisson registry;OMIN) was approved in 2015 by the French Data Protection Authority in clinical research (Commission Nationale de l'Informatique et des Libertés: number 915273) and by an independent ethics committee (Groupe Nantais d'Ethique dans le Domaine de la Santé: number 2015-01-27). Results will be discussed with associations of families affected by SUID, caregivers, funders of the registry, medical societies and researchers and will be submitted to international peer-reviewed journals and presented at international conferences.
Keywords: France; Observatoire National Des Morts Inattendues Du Nourrisson (OMIN); public health; registry; sudden infant death; sudden unexpected infant death.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
