Characterization and engineering of a plastic-degrading aromatic polyesterase
- PMID: 29666242
- PMCID: PMC5948967
- DOI: 10.1073/pnas.1718804115
Characterization and engineering of a plastic-degrading aromatic polyesterase
Abstract
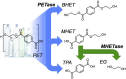
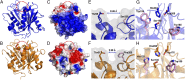
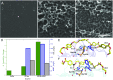
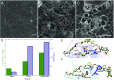
Poly(ethylene terephthalate) (PET) is one of the most abundantly produced synthetic polymers and is accumulating in the environment at a staggering rate as discarded packaging and textiles. The properties that make PET so useful also endow it with an alarming resistance to biodegradation, likely lasting centuries in the environment. Our collective reliance on PET and other plastics means that this buildup will continue unless solutions are found. Recently, a newly discovered bacterium, Ideonella sakaiensis 201-F6, was shown to exhibit the rare ability to grow on PET as a major carbon and energy source. Central to its PET biodegradation capability is a secreted PETase (PET-digesting enzyme). Here, we present a 0.92 Å resolution X-ray crystal structure of PETase, which reveals features common to both cutinases and lipases. PETase retains the ancestral α/β-hydrolase fold but exhibits a more open active-site cleft than homologous cutinases. By narrowing the binding cleft via mutation of two active-site residues to conserved amino acids in cutinases, we surprisingly observe improved PET degradation, suggesting that PETase is not fully optimized for crystalline PET degradation, despite presumably evolving in a PET-rich environment. Additionally, we show that PETase degrades another semiaromatic polyester, polyethylene-2,5-furandicarboxylate (PEF), which is an emerging, bioderived PET replacement with improved barrier properties. In contrast, PETase does not degrade aliphatic polyesters, suggesting that it is generally an aromatic polyesterase. These findings suggest that additional protein engineering to increase PETase performance is realistic and highlight the need for further developments of structure/activity relationships for biodegradation of synthetic polyesters.
Keywords: biodegradation; cutinase; plastics recycling; poly(ethylene furanoate); poly(ethylene terephthalate).
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: H.P.A., M.D.A., B.S.D., N.A.R., C.W.J., J.E.M., and G.T.B. have filed a patent application on the PETase double mutant.
Figures
References
-
- Gregory MR, Andrady AL. Plastics in the marine environment. In: Andrady AL, editor. Plastics and the Environment. Wiley; New York: 2003. pp. 379–402.
-
- Law KL, et al. Plastic accumulation in the North Atlantic subtropical gyre. Science. 2010;329:1185–1188. - PubMed
-
- Jambeck JR, et al. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. - PubMed
-
- Bergmann M, Sandhop N, Schewe I, D’Hert D. Observations of floating anthropogenic litter in the Barents Sea and Fram Strait, Arctic. Polar Biol. 2016;39:553–560.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

