Plasmodium-associated changes in human odor attract mosquitoes
- PMID: 29666273
- PMCID: PMC5939094
- DOI: 10.1073/pnas.1721610115
Plasmodium-associated changes in human odor attract mosquitoes
Abstract
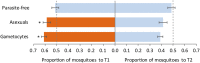
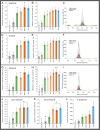
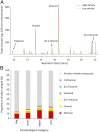
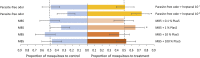
Malaria parasites (Plasmodium) can change the attractiveness of their vertebrate hosts to Anopheles vectors, leading to a greater number of vector-host contacts and increased transmission. Indeed, naturally Plasmodium-infected children have been shown to attract more mosquitoes than parasite-free children. Here, we demonstrate Plasmodium-induced increases in the attractiveness of skin odor in Kenyan children and reveal quantitative differences in the production of specific odor components in infected vs. parasite-free individuals. We found the aldehydes heptanal, octanal, and nonanal to be produced in greater amounts by infected individuals and detected by mosquito antennae. In behavioral experiments, we demonstrated that these, and other, Plasmodium-induced aldehydes enhanced the attractiveness of a synthetic odor blend mimicking "healthy" human odor. Heptanal alone increased the attractiveness of "parasite-free" natural human odor. Should the increased production of these aldehydes by Plasmodium-infected humans lead to increased mosquito biting in a natural setting, this would likely affect the transmission of malaria.
Keywords: aldehydes; disease biomarkers; host attractiveness; malaria transmission; parasite–vector–host interactions.
Conflict of interest statement
Conflict of interest statement: A.R., J.G.d.B., J.G.L., and W.T. are inventors on a patent application filed with the UK Intellectual Property Office (application no. 1805023.7).
Figures

Comment in
-
Malaria Parasites Alter Human Odor to Attract Mosquito Vectors.Trends Parasitol. 2018 Jul;34(7):547-549. doi: 10.1016/j.pt.2018.05.003. Epub 2018 Jun 1. Trends Parasitol. 2018. PMID: 29866445
References
-
- Poulin R. Parasite manipulation of host behavior: An update and frequently asked questions. Adv Study Behav. 2010;41:151–186.
-
- Day JF, Edman JD. Malaria renders mice susceptible to mosquito feeding when gametocytes are most infective. J Parasitol. 1983;69:163–170. - PubMed
-
- Coleman RE, Edman JD, Semprevivo LH. Interactions between malaria (Plasmodium yoelii) and leishmaniasis (Leishmania mexicana amazonensis): Effect of concomitant infection on host activity, host body temperature, and vector engorgement success. J Med Entomol. 1988;25:467–471. - PubMed
-
- Ferguson HM, Rivero A, Read AF. The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology. 2003;127:9–19. - PubMed
-
- Cornet S, Nicot A, Rivero A, Gandon S. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol Lett. 2013;16:323–329. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

